
Compound (A) reacts with ${[Cu{(N{H_3})_2}]^ + }$ and Tollen’s reagent, but after reaction with alc. KOH it does not give the above test. Compound (A) is:
(A) $Me - \equiv - Me$
(B) $Me - \equiv - H$
(C) $Me - C - C \equiv CH$
(D) $Ph - \equiv - H$
Answer
232.8k+ views
Hint: ${[Cu{(N{H_3})_2}]^ + }$ and Tollen’s reagent reacts on terminal alkynes and replaces the terminal H atom with Cu and Ag. While alc. KOH causes the shifting of triple to the inner carbon (adjacent bond to its earlier position).
Complete step by step answer:
-Since the compound (A) reacts with both ${[Cu{(N{H_3})_2}]^ + }$ and Tollen’s reagent ($AgN{O_3} + liq.N{H_3}$), we first need to find out which type of compound reacts with both of them. It is a terminal alkyne which reacts with both. These reactions occur in the following manner:
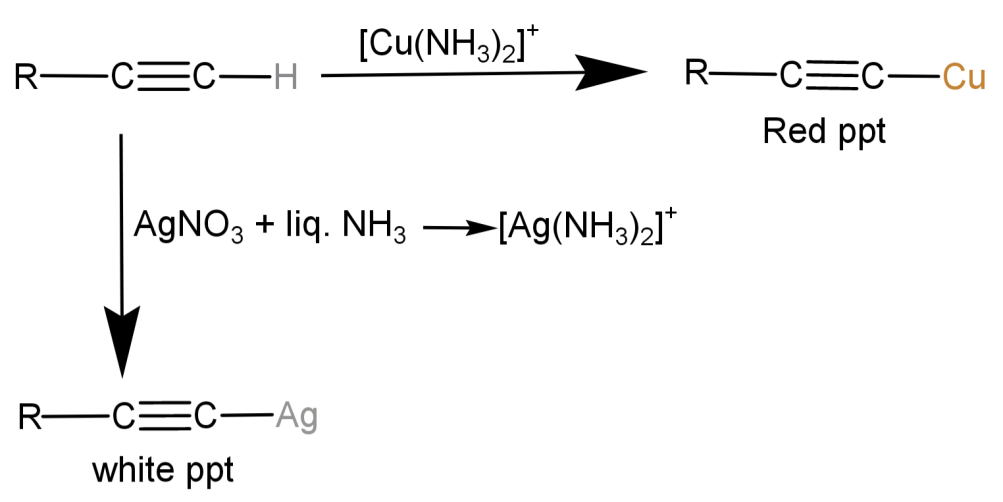
-So, we now know that the compound (A) should be a terminal alkyne.
-Also another statement is given in the question that this compound (A) does not give these above reactions after reacting with alc. KOH. The reason for this is that alc. KOH instigates isomerisation in the compound and causes the triple bond to shift to the inner carbon. For example: let us take a compound But-1-yne. On reaction with alc. KOH it becomes But-2-yne.
$C{H_3} - C{H_2} - C \equiv CH\xrightarrow{{alc.KOH}}C{H_3} - C \equiv C - C{H_3}$
Due to this, the compound becomes unable to undergo reaction with ${[Cu{(N{H_3})_2}]^ + }$ and Tollen’s reagent.
-So, from this, we can say that for alcoholic KOH to react with a terminal alkyne it should have at least 4 carbon atoms.
-In total, we have found out that this compound (A) is a terminal alkyne and has 4 or more carbon atoms in it.
-Now let us check the options.
(A)$Me - \equiv - Me$: The structure tells us that this alkyne is not terminal and hence this will not give reactions with ${[Cu{(N{H_3})_2}]^ + }$ and Tollen’s reagent.
So, this is not compound (A).
(B) $Me - \equiv - H$: This structure is a terminal alkyne but it has only 3 carbon atoms and we require a minimum of 4 carbon atoms. Hence this will give reactions with ${[Cu{(N{H_3})_2}]^ + }$ and Tollen’s reagent but not with alc.KOH.
So, this is also not compound (A).
(C) $Me - C - C \equiv CH$: Here we can see that this structure is both a terminal alkyne and has 4 carbon atoms. So I will give reactions with ${[Cu{(N{H_3})_2}]^ + }$, Tollen’s reagent and also alc. KOH.
Hence this is compound (A).
We already have our answer but just take a look at option (D) as well.
(D) $Ph - \equiv - H$: this is a terminal alkyne and also has more than 4 carbon atoms, but the shifting of triple bonds cannot occur in it. So, this will react with ${[Cu{(N{H_3})_2}]^ + }$ and Tollen’s reagent but not with alc.KOH.
So, this is also not compound (A).
Hence, the correct option is: (C)$Me - C - C \equiv CH$
Note: Alcoholic KOH does not only shift the triple bond to inner carbon in terminal alkynes, but it also causes dehydrohalogenation in alkyl halides and forms alkenes.
$C{H_3} - C{H_2}Cl\xrightarrow[{ - HCl}]{{alc.KOH}}C{H_2} = C{H_2}$
Complete step by step answer:
-Since the compound (A) reacts with both ${[Cu{(N{H_3})_2}]^ + }$ and Tollen’s reagent ($AgN{O_3} + liq.N{H_3}$), we first need to find out which type of compound reacts with both of them. It is a terminal alkyne which reacts with both. These reactions occur in the following manner:

-So, we now know that the compound (A) should be a terminal alkyne.
-Also another statement is given in the question that this compound (A) does not give these above reactions after reacting with alc. KOH. The reason for this is that alc. KOH instigates isomerisation in the compound and causes the triple bond to shift to the inner carbon. For example: let us take a compound But-1-yne. On reaction with alc. KOH it becomes But-2-yne.
$C{H_3} - C{H_2} - C \equiv CH\xrightarrow{{alc.KOH}}C{H_3} - C \equiv C - C{H_3}$
Due to this, the compound becomes unable to undergo reaction with ${[Cu{(N{H_3})_2}]^ + }$ and Tollen’s reagent.
-So, from this, we can say that for alcoholic KOH to react with a terminal alkyne it should have at least 4 carbon atoms.
-In total, we have found out that this compound (A) is a terminal alkyne and has 4 or more carbon atoms in it.
-Now let us check the options.
(A)$Me - \equiv - Me$: The structure tells us that this alkyne is not terminal and hence this will not give reactions with ${[Cu{(N{H_3})_2}]^ + }$ and Tollen’s reagent.
So, this is not compound (A).
(B) $Me - \equiv - H$: This structure is a terminal alkyne but it has only 3 carbon atoms and we require a minimum of 4 carbon atoms. Hence this will give reactions with ${[Cu{(N{H_3})_2}]^ + }$ and Tollen’s reagent but not with alc.KOH.
So, this is also not compound (A).
(C) $Me - C - C \equiv CH$: Here we can see that this structure is both a terminal alkyne and has 4 carbon atoms. So I will give reactions with ${[Cu{(N{H_3})_2}]^ + }$, Tollen’s reagent and also alc. KOH.
Hence this is compound (A).
We already have our answer but just take a look at option (D) as well.
(D) $Ph - \equiv - H$: this is a terminal alkyne and also has more than 4 carbon atoms, but the shifting of triple bonds cannot occur in it. So, this will react with ${[Cu{(N{H_3})_2}]^ + }$ and Tollen’s reagent but not with alc.KOH.
So, this is also not compound (A).
Hence, the correct option is: (C)$Me - C - C \equiv CH$
Note: Alcoholic KOH does not only shift the triple bond to inner carbon in terminal alkynes, but it also causes dehydrohalogenation in alkyl halides and forms alkenes.
$C{H_3} - C{H_2}Cl\xrightarrow[{ - HCl}]{{alc.KOH}}C{H_2} = C{H_2}$
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




