
What is the chief product obtained when n−butane is treated with bromine in the presence of light at 403K?
A. 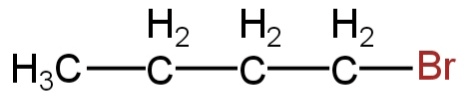
B. 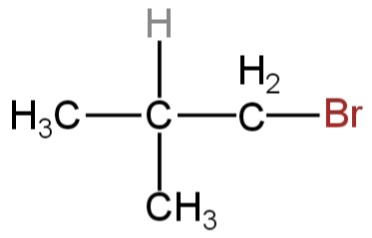
C. 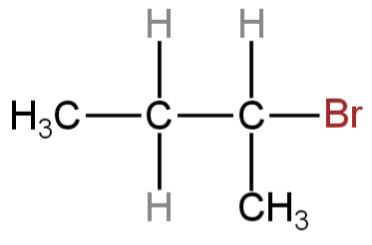
D. 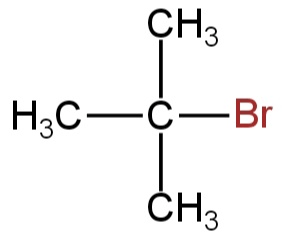
Answer
233.1k+ views
Hint: The bromination of n-butane is a substitution reaction. A substitution reaction is a chemical reaction that involves a portion of the reacting molecules substituting an atom or a group of atoms on a hydrocarbon or a compound derived from a hydrocarbon.
Complete Step by Step Answer:
A halogenation reaction is a chemical reaction involving a substrate and a halogen and one or more halogen atoms are combined with the molecules of the substrate.
Halogenation of an alkane yields a hydrocarbon derivative with one or more hydrogen atoms replaced by halogen atoms.
Alkanes are unreactive compounds towards substitution. But free radical halogenation involves substitution.
This reaction occurs in three steps.
1. Initiation Step
The Br-Br bond undergoes homolytic cleavage in presence of UV light and results in two bromine free radicals.
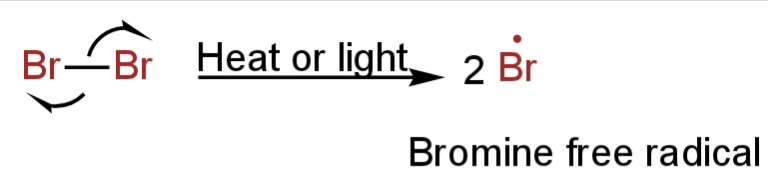
Image: Initiation step
2. Propagation Step
The bromine radical takes a hydrogen atom from n-butane and produces the butyl radical.
This butyl radical formed is of two types:
primary and secondary butyl radical.
The secondary free radical is more stable due to hyperconjugation.
Here the sec-butyl free radical has 5 α-Hydrogen atoms whereas the primary butyl free radical has only four.
The sec-butyl free radical then abstracts a bromine free radical from a bromine molecule and sec-butyl chloride or 2-butyl chloride is formed.
Butyl chloride is formed but 2-butyl chloride is the major product.
This step of propagation also restores a bromine atom.
These steps happen numerous times until termination happens.
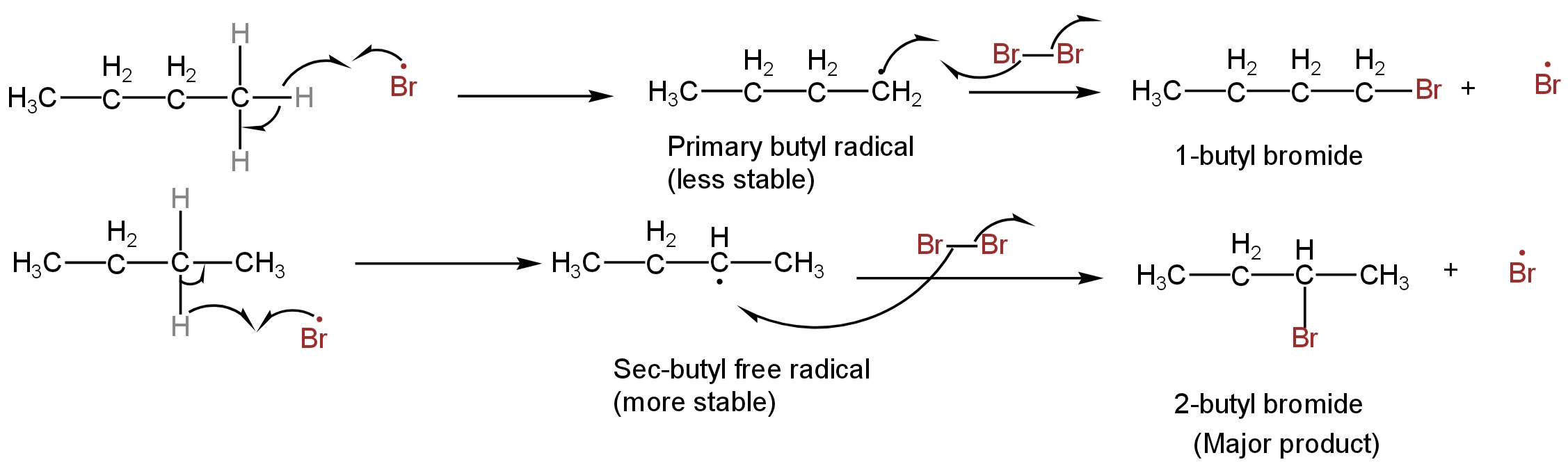
Image: Propagation step
3. Termination step
It takes place when a bromine atom reacts with another bromine atom to yield bromine, or a bromine atom can react with a primary butyl free radical to form 2-butyl chloride which is a minor product.
The sec-butyl radical and primary butyl radicals combine to form a long carbon chain.
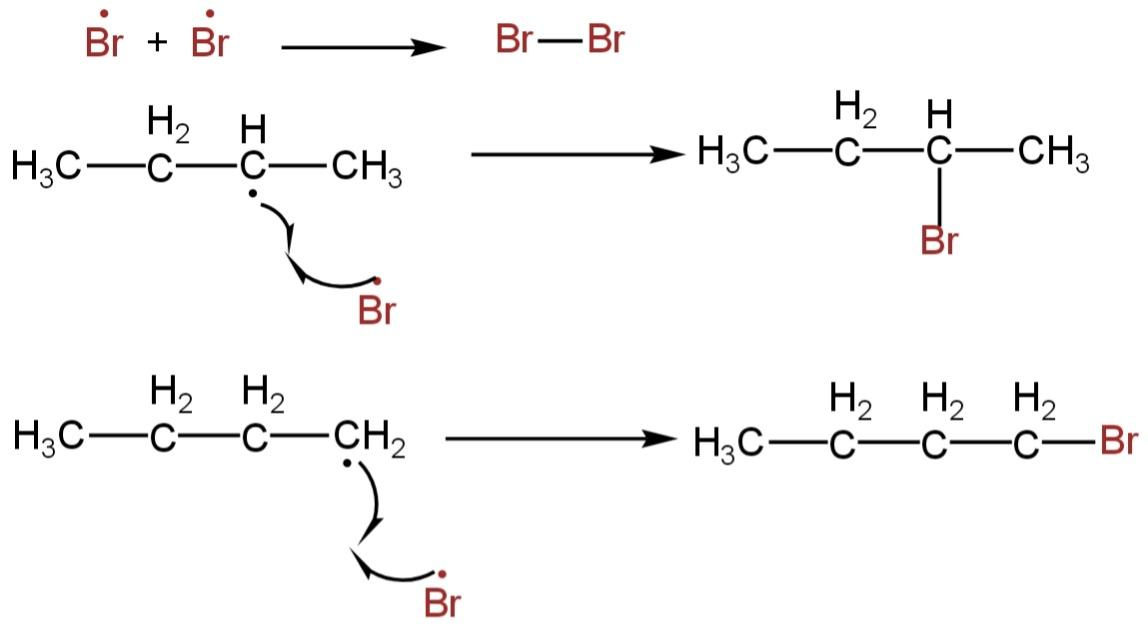
Image: Termination step
So, 2-butyl bromide is formed as a major product when n−butane is treated with bromine in the presence of light at 403K.
So, option C is correct.
Note: This reaction does not cease at the last step, however, as the brominated butane may undergo a reaction with extra bromine to yield polybrominated products. With the regulation of the reaction conditions and the ratio of bromine to n-butane, we can prefer the yield of one or more brominated butane products.
Complete Step by Step Answer:
A halogenation reaction is a chemical reaction involving a substrate and a halogen and one or more halogen atoms are combined with the molecules of the substrate.
Halogenation of an alkane yields a hydrocarbon derivative with one or more hydrogen atoms replaced by halogen atoms.
Alkanes are unreactive compounds towards substitution. But free radical halogenation involves substitution.
This reaction occurs in three steps.
1. Initiation Step
The Br-Br bond undergoes homolytic cleavage in presence of UV light and results in two bromine free radicals.

Image: Initiation step
2. Propagation Step
The bromine radical takes a hydrogen atom from n-butane and produces the butyl radical.
This butyl radical formed is of two types:
primary and secondary butyl radical.
The secondary free radical is more stable due to hyperconjugation.
Here the sec-butyl free radical has 5 α-Hydrogen atoms whereas the primary butyl free radical has only four.
The sec-butyl free radical then abstracts a bromine free radical from a bromine molecule and sec-butyl chloride or 2-butyl chloride is formed.
Butyl chloride is formed but 2-butyl chloride is the major product.
This step of propagation also restores a bromine atom.
These steps happen numerous times until termination happens.

Image: Propagation step
3. Termination step
It takes place when a bromine atom reacts with another bromine atom to yield bromine, or a bromine atom can react with a primary butyl free radical to form 2-butyl chloride which is a minor product.
The sec-butyl radical and primary butyl radicals combine to form a long carbon chain.

Image: Termination step
So, 2-butyl bromide is formed as a major product when n−butane is treated with bromine in the presence of light at 403K.
So, option C is correct.
Note: This reaction does not cease at the last step, however, as the brominated butane may undergo a reaction with extra bromine to yield polybrominated products. With the regulation of the reaction conditions and the ratio of bromine to n-butane, we can prefer the yield of one or more brominated butane products.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




