




How to Interpret and Draw Wedge Dash Structures for Molecules
The Wedge Dash Representation is a fundamental concept in JEE Main Chemistry, especially in stereochemistry and the understanding of three-dimensional molecular structures. This method visually distinguishes whether bonds or groups attached to a central atom, usually carbon, are projecting towards or away from the observer, crucial for recognizing chiral centers and stereoisomers. For exam clarity, understanding this visual system links directly with the mechanism of isomerism, chirality, and molecular recognition found in more complex organic and biomolecular scenarios.
In three-dimensional representations, a solid wedge or thick black line indicates a bond projecting out towards you, above the plane of the paper. Meanwhile, a dashed wedge shows a bond pointing away, behind the plane. Simple straight lines represent bonds lying directly on the plane of the paper. Mastery of this technique is essential for differentiating optical isomers and understanding real molecules beyond flat Lewis structures, preparing you for the visualization demands of JEE Main Chemistry.
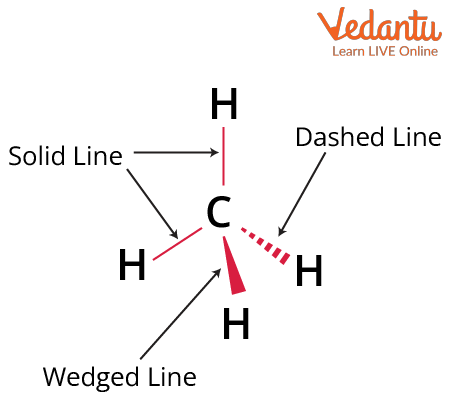
Several related techniques exist to condense molecular geometry onto paper beyond the Wedge Dash Representation. The Fischer Projection and the Sawhorse Representation are common, with the latter useful for visualizing adjacent carbon atoms. The Chemical Bonding principles and the Molecular Orbital Theory also reinforce how molecular shape and bonding relate to stereochemistry and three-dimensional arrangements.
In competitive exams like JEE Main, it’s crucial to convert between these formats efficiently. Some tips for translation:
- Wedge Dash to Fischer: Place the most oxidized carbon at the top, horizontal bonds as wedges, and vertical bonds as dashes.
- Wedge Dash to Newman Projection: Look down the bond axis, with front carbon as a dot and rear as a circle, projecting substituents at defined angles.
Common traps include misplacing the direction of a wedge or dash and confusing which group is in front or behind. Always double-check priorities when assigning R or S configurations. Assign priority based on atomic numbers (highest first), and remember the sequence: a clockwise order with lowest priority group on the dashed bond gives R configuration; anticlockwise gives S, as defined by the Cahn-Ingold-Prelog system.
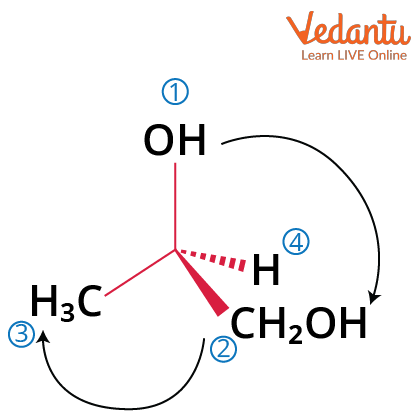
Wedge dash structures quickly communicate molecular chirality—especially useful for identifying enantiomers and diastereomers. Accurate sketching of these forms supports NMR assignments, nucleophilic substitution reaction mechanisms, and the study of hybridization and covalent bonding in advanced chapters.
Comparing different 3-D representations is a common JEE task. The following table provides a concise reference to the major molecular depiction systems:
| Representation | Key Features | Best for |
|---|---|---|
| Wedge Dash | Solid/dashed lines for bond direction | Chiral carbons, basic stereochemistry |
| Fischer Projection | Crossed lines; vertical bonds away, horizontal towards | Carbohydrates, amino acids |
| Sawhorse | Diagonal draw for adjacent carbons | Conformations, relative group orientation |
| Newman Projection | Circle/dot; view down bond axis | Conformers, torsion angles |
For comprehensive JEE Main preparation, explore Chemical Bonding and Molecular Structure and Hybridization of Carbon for deeper context. Mastering wedge dash and alternative projections strengthens your ability to tackle questions on isomerism, stereochemistry, and mechanisms across organic chemistry. Apply this knowledge when analyzing types of chemical bonds and determining the properties of isomeric compounds.
- Practice regularly by drawing simple molecules in wedge dash and converting them to Fischer or Newman style.
- Learn to assign R/S configuration rapidly for each chiral center using wedge dash figures.
- Reinforce your revision with solved JEE previous year questions using these representations.
- Use structural visualization skills to solve reaction mechanism predictions.
- Study the differences in physical and chemical properties between isomers as seen through these diagrams.
Vedantu’s focused study materials and revision notes use the wedge dash representation systematically, helping you connect spatial visualisation to the logic and language of exam questions. Strategic command of these representations ensures you avoid common mistakes and strengthens your scores in both organic structure elucidation and stereochemistry problems in JEE Main Chemistry.
FAQs on Understanding Wedge Dash Representation in Organic Chemistry
1. What is wedge dash representation in chemistry?
Wedge dash representation is a diagrammatic method used to show the three-dimensional arrangement of atoms in a molecule on a two-dimensional surface. It helps visualize stereochemistry and distinguish between bonds that lie in the plane, project outwards, or go behind the plane.
Key points:
- Solid wedge (△): bond projects out of the plane (towards observer)
- Dashed wedge (---): bond goes behind the plane (away from observer)
- Normal lines: bonds lie in the plane of the paper
2. Why is wedge dash representation important in organic chemistry?
Wedge dash representation is crucial in organic chemistry because it allows students to clearly visualize the spatial arrangement of atoms and determine stereochemistry.
Major purposes include:
- Distinguishing between enantiomers and diastereomers
- Showing the 3D orientation of substituents
- Predicting physical and chemical properties of stereoisomers
3. How do you draw a molecule using wedge dash notation?
To draw a molecule using wedge dash notation, start with the central atom and clearly indicate the position of each substituent.
Follow these steps:
- Draw the central atom.
- Use a normal line for bonds in the plane of the paper.
- Draw a solid wedge to show a bond projecting outward.
- Use a hashed (dashed) wedge for bonds going behind the plane.
- Assign substituents at appropriate 3D positions to reflect actual molecular geometry.
4. What is the difference between Fischer projection and wedge dash representation?
The wedge dash representation and Fischer projection are both ways to depict the structure of molecules, but they differ in how they show 3D orientation:
Wedge dash:
- Depicts true 3D geometry
- Uses wedges/dashes for out-of-plane and in-plane bonds
- 2D representation used mainly for sugars and amino acids
- Horizontal lines: bonds towards viewer; Vertical lines: bonds away from viewer
5. What is the significance of wedge and dash in showing stereochemistry?
Wedge and dash notation is significant because it helps differentiate between stereoisomers by displaying the exact spatial arrangement of atoms or groups.
It is especially important for:
- Identifying chiral centers
- Representing optical activity
- Distinguishing between R- and S- configurations
6. Give an example of wedge dash representation for methane (CH4).
The wedge dash structure of methane (CH4) shows the tetrahedral arrangement of hydrogens around carbon.
Representation:
- Central carbon atom
- Two H atoms in the plane (normal lines)
- One H atom coming out (solid wedge)
- One H atom going behind (dashed line)
7. How does wedge dash representation help identify chiral centers?
Wedge dash representation clearly demonstrates the spatial arrangement of substituents, enabling identification of chiral centers.
For a carbon to be chiral:
- It must have four different substituents
- The orientation shown must be non-superimposable on its mirror image
8. What are the conventions of drawing wedge and dash bonds?
The conventions for drawing wedge and dash bonds are standardized to represent 3D orientation:
- Solid wedge: out of the paper (towards the viewer)
- Dashed wedge: behind the plane (away from the viewer)
- Normal line: in the plane of the paper
9. Which chapters in CBSE chemistry use wedge dash representation?
Wedge dash representation is commonly used in several chapters of CBSE Class 11 and 12 Chemistry, especially:
- Organic Chemistry – Some Basic Principles and Techniques
- Stereochemistry
- Haloalkanes and Haloarenes
10. Can wedge dash representation be used for any molecule?
Wedge dash representation can be used for any molecule where the 3D arrangement of atoms needs to be clarified,
but it is particularly important for molecules with chiral centers or multiple stereoisomers.
- Best suited for tetrahedral centers
- Helps represent complicated organic molecules























