




Introduction
Electrophilic aromatic substitution occurs in many substituted benzene rings. Each substituent raises or lowers the electron density in the benzene ring, which influences the path of electrophilic aromatic replacement. The addition of electron density to the ring makes benzene more stable. Electrophiles are more likely to react with electron-rich compounds. The removal of electrons from the ring makes benzene electron-deficient, and, thus, they are less likely to react with an electrophile. Electrophilic substitution on benzene that has already been substituted generates isomers, some of which are preferred over others.
Effect of Substituents on the Reactivity of Benzene
The most common reaction in benzene and many of its derivatives are electrophilic substitution reactions. Organic reactions in which an electrophile replaces an atom attached to an aromatic ring are known as ‘electrophilic aromatic substitution reactions’. These reactions typically involve the replacement of a hydrogen atom from a benzene ring with an electrophile.
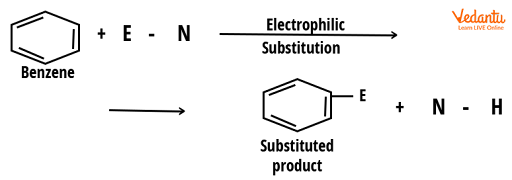
Electrophilic Aromatic Substitution Reaction
Two aspects of the electrophilic aromatic substitution reaction of benzene are influenced by a substituent, which are:
1. Reaction rate: A substituted benzene reacts faster or slower than pure benzene.
2. The orientation: The new group is orthogonal, metagonal, or paragonal to the existing substituent. The position of the second incoming substituent is determined by the identity of the first substituent.
Electron-Donating Group (EDG)
An electron-donating group (EDG) increases electron density in a molecule via the carbon atom to which it is bonded. EDGs alter a molecule's reactivity by increasing electron density on adjacent carbon atoms: EDGs make nucleophiles stronger. A nucleophilic centre with EDGs is even more electron-rich and ready to attack electrophilic sites.
Carbon centres become weaker electrophiles and less reactive to nucleophiles as a result of EDGs because any (partial) positive charge is minimised or nullified if the EDG is strong enough. Groups with lone pairs to donate, such as an oxygen anion, are examples of good electron-donating groups.
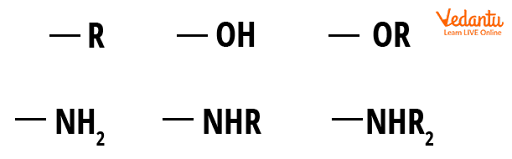
Examples of Good Electron-Donating Groups.
Electron-Withdrawing Group
An Electron-Withdrawing Group (EWG) is a group that reduces electron density in a molecule by being bonded to a carbon atom. EWGs alter a molecule's reactivity by reducing electron density on adjacent carbon atoms:
As the electron-withdrawing effect makes any carbon centre even more electron-deficient than before, EWGs strengthen the electrophiles. EWGs reduce the reactivity of any nucleophilic species for the same reason that they strengthen electrophiles. Nucleophiles require electron density in order to react with electrophiles. The strongest EWGs are those with pi-bonds to electronegative atoms:
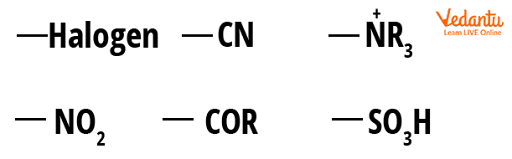
Examples of Electron-Withdrawing Group
Reactivity: Activation and Deactivation
Since benzene acts as a nucleophile in electrophilic aromatic substitution, the substituents that make benzene more electron-rich can speed up the reaction. The process can be slowed by substituents that make benzene more electron-poor or which draw electron density away from the aromatic ring.
Deactivating groups are the groups involved in this reaction. The reaction is aided by substitutes that easily contribute electron density to the ring or efficiently stabilise the cationic intermediate. In this reaction, these groups are known as ‘activating groups’.
These groups' roles are determined by their electronic interactions with the electrons in the ring. Some groups (for example H2N-, HO-, RO-) have lone pairs and act as -donors, adding electron density to the benzene ring via resonance. This is known as the +R (for resonance) effect and it activates the ring, causing it to undergo electrophilic aromatic substitution.
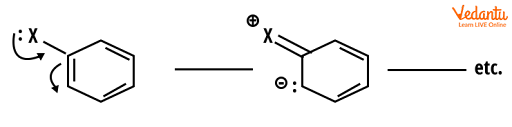
Reactivity example -activation of benzene ring
Other groups have an electronegative atom attached via a -bond (e.g., C=O), which causes the group to be electron-withdrawing. These groups function as -acceptors, attracting electron density away from the ring through resonance. This is known as the –R effect and it deactivates the ring for electrophilic aromatic substitution.
Some groups only act through sigma bonds via the inductive effect (I), which is purely based on electronegativity and lacks resonance. These effects are typically less pronounced than resonance effects but are still significant.
As sp3 carbon is less electronegative than a sp2 carbon, a methyl or similar sp3, the alkyl (R) group can act as a -donor, introducing extra electron density into the ring and resulting in a +I effect (activating). Groups based on more electronegative atoms (O, F, Cl) can act as -acceptors, drawing electron density away from the ring via a simple inductive effect caused by the substituent's electronegativity. This deactivates the ring and is known as the –I effect.
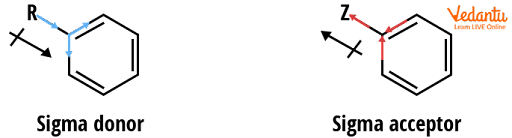
Reactivity Example - sigma donor and acceptor
Directing Effects
Substituents on the benzene ring influence the regiochemistry of the reaction in addition to the speed of the reaction. A new substituent can attach to the benzene ring in three different positions relative to the original substituent. Substitution could occur in five different positions around the ring. There are two ortho– and two meta–positions to the initial substituent.
Activating Groups (Ortho or Para Directors)
When substituents with an unshared pair of electrons, such as -OH, have an unshared pair of electrons, the resonance effect is stronger than the inductive effect, making these substituents stronger activators, as the resonance effect directs the electron toward the ring.

Activating groups (ortho or para directors)
The ortho and para carbons are more likely to react with the electrophile because the extra electron density is localized on them. When s-electrons are pushed towards the ring, the inductive effects of alkyl groups activate in the direction of the ortho or para substitution.
Deactivating Group (Meta Directors)
The deactivating groups deactivate the ring by inducing electron density away from the ring in the presence of an electronegative atom.
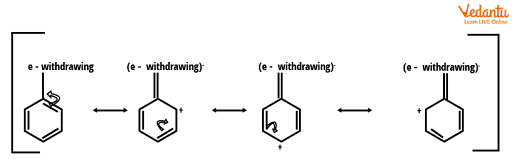
Deactivating groups (meta directors)
When electron density is removed from the ring, the carbons at the ortho and para positions have a partial positive charge, which is unfavourable for the electrophile. Thus, the electrophile attacks the carbon at the meta positions. The deactivating group that directs to the ortho or para substitution is an exception for halogens. Despite having an unpaired pair of electrons, halogens deactivate the ring via inductive effect rather than resonance. The unpaired pair of electrons are donated to the ring, but the halogens' electronegativity pulls the s-electrons away from the ring.
Conclusion
Many substituted benzene rings undergo electrophilic aromatic substitution. Each substituent changes the electron density in the benzene ring, influencing the path of electrophilic aromatic substitution. Effect of substituents on the reactivity of benzene has two aspects of the electrophilic aromatic substitution reaction of benzene. First one is the reaction rate, which determines whether a substituted benzene reacts faster or slower than pure benzene. The second is the benzene ring orientation. Benzene is a planar molecule with electrons delocalized above and below the plane of the ring. As a result, it helps the process of electrophilic substitution reactions.
FAQs on Effect of Substituent on Reactivity of Benzene for JEE
1. Why is phenol more reactive than benzene in electrophilic substitution reactions?
The donation of the lone pair of oxygen into the ring system raises the electron density around the ring. As a result, the ring is much more reactive than in benzene. The intermediate carbocation has a higher degree of resonance stability. We can also say that the presence of the OH group in phenol, which is an electron-donating group, increases the electron density by +R effects on the benzene ring. Hence, with the presence of an activating group on phenol that is absent in benzene, phenol is more reactive than benzene in an electrophilic substitution reaction.
2. Which is most reactive in electrophilic substitution?




Option (D) phenol, because of the +R Effect of the -OH group, which increases the reactivity in aromatic electrophilic substitution reactions. The donation of the lone pair of oxygen into the ring system raises the electron density around the ring. As a result, the ring is much more reactive than in benzene. The second most reactive is chlorobenzene due to the presence of an activating group and the third one is benzene which has no attached group. The least reactive among these is nitrobenzene, which is due to the presence of a deactivating group.
3. Why does benzene assist the process of electrophilic substitution reactions but struggle with nucleophilic substitutions?
Benzene is a planar molecule with delocalized electrons above and below the plane of the ring. As a result, it is electron-dense. As a result, it is very appealing to electron-deficient species, i.e., electrophiles. As a result, it easily performs electrophilic substitution reactions. Nucleophiles are electron-rich. Nucleophilic attack on the benzene ring is difficult due to the presence of an electron cloud of delocalized electrons. As a result, they are repulsed by benzene. As a result, benzene undergoes nucleophilic substitutions with difficulty.
























