
How many carbon-hydrogen bond orbitals are available for overlap with the vacant p-orbital in ethyl carbocation?
(a) 0
(b) 3
(c) 5
(d) 6
Answer
240.3k+ views
Hint: Carbocation is a species in which the carbon atom has a positive charge and can form three bonds. Stability of carbocation depends on the number of carbon atoms and functional groups attached to it.
Complete step by step solution:
-Ethyl carbocation is ethane in which one of the hydrogen is missing from one carbon and it has a positive charge on that carbon atom. The structure of ethyl carbocation is as shown in the figure below
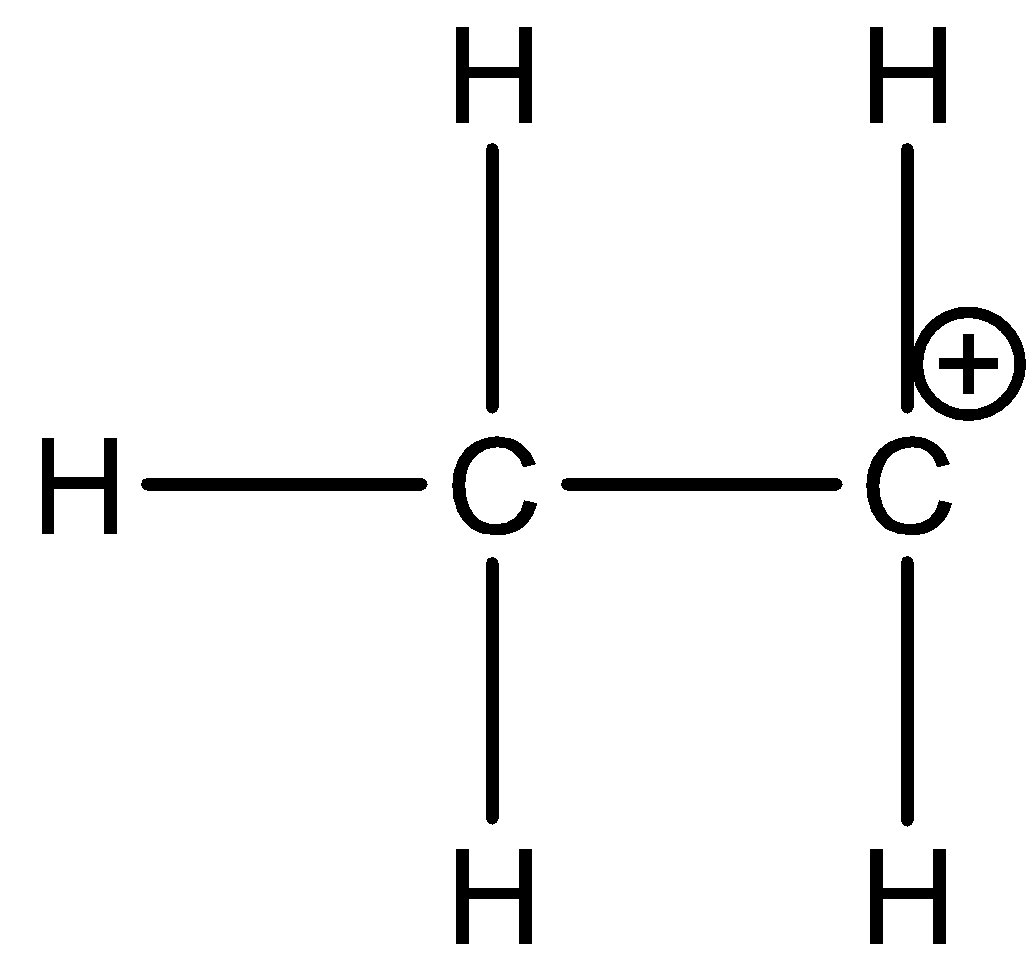
-Now let us see how many p orbitals are there vacant in the carbocation.
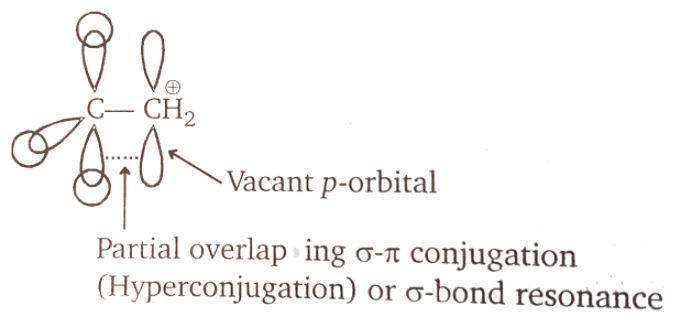
There are two vacant orbitals. The carbon next to carbocation has carbon-hydrogen bonds. All the three are available for the overlap with the vacant orbital of the carbocation. The vacant p-orbital forms a partial overlap giving a partial sigma-pi conjugation. This can also lead to hyperconjugation. Hyper conjugation is the interaction of the electrons in a sigma bond (C-H or C-C) with adjacent empty or partially filled p orbital or pi orbital and it gives an extended molecular orbital which increases the stability of the system. It is not resonance.
Hence, the 3 carbon-hydrogen bonds are available for overlap with the vacant p-orbital of the ethyl carbocation. The correct answer to the question is option (b).
Note: Due to the vacant p orbital, carbocation is electron deficient. Because of this, it can act as an electrophile. Carbocation has a trigonal planar shape.
Complete step by step solution:
-Ethyl carbocation is ethane in which one of the hydrogen is missing from one carbon and it has a positive charge on that carbon atom. The structure of ethyl carbocation is as shown in the figure below

-Now let us see how many p orbitals are there vacant in the carbocation.

There are two vacant orbitals. The carbon next to carbocation has carbon-hydrogen bonds. All the three are available for the overlap with the vacant orbital of the carbocation. The vacant p-orbital forms a partial overlap giving a partial sigma-pi conjugation. This can also lead to hyperconjugation. Hyper conjugation is the interaction of the electrons in a sigma bond (C-H or C-C) with adjacent empty or partially filled p orbital or pi orbital and it gives an extended molecular orbital which increases the stability of the system. It is not resonance.
Hence, the 3 carbon-hydrogen bonds are available for overlap with the vacant p-orbital of the ethyl carbocation. The correct answer to the question is option (b).
Note: Due to the vacant p orbital, carbocation is electron deficient. Because of this, it can act as an electrophile. Carbocation has a trigonal planar shape.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Difference Between Crystalline and Amorphous Solid: Table & Examples

Types of Solutions in Chemistry: Explained Simply

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Notes Class 11 Chemistry Chapter 9 - Hydrocarbons - 2025-26

CBSE Notes Class 11 Chemistry Chapter 5 - Thermodynamics - 2025-26

CBSE Notes Class 11 Chemistry Chapter 6 - Equilibrium - 2025-26

Inductive Effect and Its Role in Acidic Strength




