
Bottles containing \[{C_6}{H_5}I\] and \[{C_6}{H_5}C{H_2}I\] lost their original labels. They are labelled A and B for testing. A and B were separately taken in a test tube and boiled with \[NaOH\] solution. The end solution in each tube was made acidic with dilute \[HN{O_3}\] and then some \[AgN{O_3}\] solution was added. Substance B gave a yellow precipitate. Which one of the following statements is true for this experiment?
A. A was \[{C_6}{H_5}I\]
B. A was \[{C_6}{H_5}C{H_2}I\]
C. B was \[{C_6}{H_5}I\]
D. Addition of \[HN{O_3}\]was unnecessary
Answer
232.8k+ views
Hint: Reacting iodobenzene (\[{C_6}{H_5}I\]) and benzyl iodide (\[{C_6}{H_5}C{H_2}I\]) with sodium hydroxide (\[NaOH\]) solution results in the nucleophilic substitution reaction (via the\[{S_N}1\]pathway) of only one of them. That will be the compound giving yellow precipitate with silver nitrate (\[AgN{O_3}\]).
Complete Step by Step Solution:
The given compounds are iodobenzene (\[{C_6}{H_5}I\]) and benzyl iodide (\[{C_6}{H_5}C{H_2}I\]). Their structures are given below:
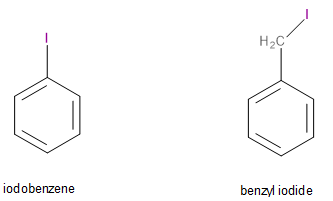
Image: Structures of iodobenzene and benzyl iodide
On boiling benzyl iodide with sodium hydroxide solution, the iodine atom gets substituted by a hydroxyl group (\[ - OH\]). This is a nucleophilic substitution reaction that takes place via the\[{S_N}1\]mechanism.
The first step involves the iodine atom leaving the molecule as an iodide ion (\[{I^ - }\]) leaving behind a benzyl carbocation as shown below:
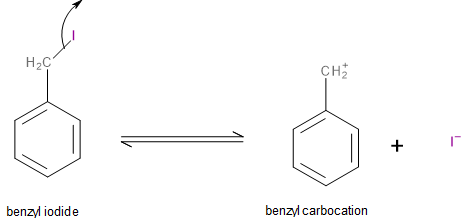
Image: Step 1
The second step involves the nucleophilic attack of the hydroxide ion (\[O{H^ - }\]) from sodium hydroxide.
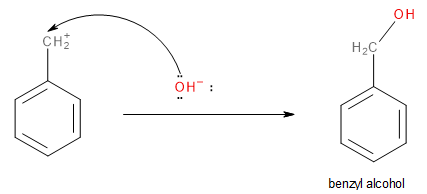
Image: Step 2
The iodide ion liberated in step 1 reacts with silver nitrate (\[AgN{O_3}\]) solution to form a pale-yellow precipitate of silver iodide (\[AgI\]).
\[{I^ - }(aq) + AgN{O_3}(aq) \to AgI(s) + NO_3^ - (aq)\]
This process does not occur in the case of iodobenzene because the carbocation formed in this case is an unstable phenyl carbocation. It is unstable because the positive charge exists on a doubly bonded, \[s{p^2}\]hybridised carbon. Carbocations become stabilised when they are attached to groups that can donate an electron pair into their empty p-orbitals. In the case of phenyl carbocation, the\[s{p^2}\]nature of the positively charged carbon atom prevents it from donating an electron pair and stabilising the carbocation.
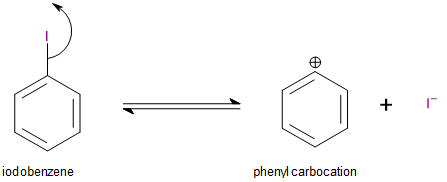
Image: Dissociation of iodobenzene to form a phenyl carbocation
On the other hand, the benzyl carbocation formed in the case of benzyl chloride is resonance stabilised. Thus, it can undergo the\[{S_N}1\]reaction with\[NaOH\].

Image: Resonating structures of benzyl carbocation
Thus, iodobenzene (\[{C_6}{H_5}I\]) is compound A and benzyl iodide (\[{C_6}{H_5}C{H_2}I\]) is compound B.
Thus, option A is correct.
Note: The answer to this question entirely hinges on the various factors that promote or inhibit\[{S_N}1\]reactions such as the stability of carbocations. Addition of dilute \[HN{O_3}\]also factors into this since\[HN{O_3}\]is protic and we know that protic environments promote\[{S_N}1\]reactions by helping make the carbocation intermediate stable.
Complete Step by Step Solution:
The given compounds are iodobenzene (\[{C_6}{H_5}I\]) and benzyl iodide (\[{C_6}{H_5}C{H_2}I\]). Their structures are given below:

Image: Structures of iodobenzene and benzyl iodide
On boiling benzyl iodide with sodium hydroxide solution, the iodine atom gets substituted by a hydroxyl group (\[ - OH\]). This is a nucleophilic substitution reaction that takes place via the\[{S_N}1\]mechanism.
The first step involves the iodine atom leaving the molecule as an iodide ion (\[{I^ - }\]) leaving behind a benzyl carbocation as shown below:

Image: Step 1
The second step involves the nucleophilic attack of the hydroxide ion (\[O{H^ - }\]) from sodium hydroxide.

Image: Step 2
The iodide ion liberated in step 1 reacts with silver nitrate (\[AgN{O_3}\]) solution to form a pale-yellow precipitate of silver iodide (\[AgI\]).
\[{I^ - }(aq) + AgN{O_3}(aq) \to AgI(s) + NO_3^ - (aq)\]
This process does not occur in the case of iodobenzene because the carbocation formed in this case is an unstable phenyl carbocation. It is unstable because the positive charge exists on a doubly bonded, \[s{p^2}\]hybridised carbon. Carbocations become stabilised when they are attached to groups that can donate an electron pair into their empty p-orbitals. In the case of phenyl carbocation, the\[s{p^2}\]nature of the positively charged carbon atom prevents it from donating an electron pair and stabilising the carbocation.

Image: Dissociation of iodobenzene to form a phenyl carbocation
On the other hand, the benzyl carbocation formed in the case of benzyl chloride is resonance stabilised. Thus, it can undergo the\[{S_N}1\]reaction with\[NaOH\].

Image: Resonating structures of benzyl carbocation
Thus, iodobenzene (\[{C_6}{H_5}I\]) is compound A and benzyl iodide (\[{C_6}{H_5}C{H_2}I\]) is compound B.
Thus, option A is correct.
Note: The answer to this question entirely hinges on the various factors that promote or inhibit\[{S_N}1\]reactions such as the stability of carbocations. Addition of dilute \[HN{O_3}\]also factors into this since\[HN{O_3}\]is protic and we know that protic environments promote\[{S_N}1\]reactions by helping make the carbocation intermediate stable.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




