
B−H−B bridge in \[{{B}_{2}}{{H}_{6}}\]is formed by sharing of:
(A) 2 electrons
(B) 4 electrons
(C) 1 electrons
(D) 3 electrons
Answer
233.1k+ views
Hint: To answer this question, we should first draw the structure of diborane. When we draw the structure of a diborane, then only we can tell the number of electrons in the BHB bridge.
Complete step by step solution:
> We should first know about diborane. It has the chemical formula of \[{{B}_{2}}{{H}_{6}}\]. - It is the chemical compound consisting of boron and hydrogen with the formula \[{{B}_{2}}{{H}_{6}}\]. So first we will draw the structure of Diborane. We should carefully draw the structure of Diborane. We should know that four hydrogen in diborane are attached to terminals, while two hydrogens act as bridges between the boron centers. We should understand that the lengths of the B-H bridge bonds and the B-H terminal bonds are 1.33 and 1.19 Å, respectively. This difference in bond lengths reflects the difference in their strengths, the B-H bridge bonds being relatively weaker.
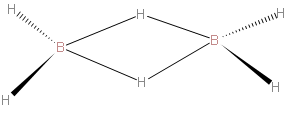
> The above structure represented is of \[{{B}_{2}}{{H}_{6}}\]..
As we draw the structure of\[{{B}_{2}}{{H}_{6}}\], it describes the bonds between boron and the terminal hydrogen atoms as traditional 2-center, 2-electron covalent bonds. The bonding between the boron atoms and the bridging hydrogen atoms is, however, different from that in molecules such as hydrocarbons. We know that boron uses two electrons in bonding to the terminal hydrogen atoms, and has one valence electron remaining for additional bonding. The bridging hydrogen atoms provide one electron each. The \[{{B}_{2}}{{H}_{2}}\] ring is held together by four electrons which form two 3-center 2-electron bonds. This type of bond is sometimes called a 'banana bond'.
> So, from the above discussion, we came to know that the B-H-B bridge in \[{{B}_{2}}{{H}_{6}}\] is formed by sharing two electrons. Therefore correct option is (A).
Note:We should note that diborane can be used as a rocket propellant. The complete combustion of diborane is strongly exothermic. But we should also know that this shows the similarity with carbon monoxide and doesn’t burn completely.
Complete step by step solution:
> We should first know about diborane. It has the chemical formula of \[{{B}_{2}}{{H}_{6}}\]. - It is the chemical compound consisting of boron and hydrogen with the formula \[{{B}_{2}}{{H}_{6}}\]. So first we will draw the structure of Diborane. We should carefully draw the structure of Diborane. We should know that four hydrogen in diborane are attached to terminals, while two hydrogens act as bridges between the boron centers. We should understand that the lengths of the B-H bridge bonds and the B-H terminal bonds are 1.33 and 1.19 Å, respectively. This difference in bond lengths reflects the difference in their strengths, the B-H bridge bonds being relatively weaker.

> The above structure represented is of \[{{B}_{2}}{{H}_{6}}\]..
As we draw the structure of\[{{B}_{2}}{{H}_{6}}\], it describes the bonds between boron and the terminal hydrogen atoms as traditional 2-center, 2-electron covalent bonds. The bonding between the boron atoms and the bridging hydrogen atoms is, however, different from that in molecules such as hydrocarbons. We know that boron uses two electrons in bonding to the terminal hydrogen atoms, and has one valence electron remaining for additional bonding. The bridging hydrogen atoms provide one electron each. The \[{{B}_{2}}{{H}_{2}}\] ring is held together by four electrons which form two 3-center 2-electron bonds. This type of bond is sometimes called a 'banana bond'.
> So, from the above discussion, we came to know that the B-H-B bridge in \[{{B}_{2}}{{H}_{6}}\] is formed by sharing two electrons. Therefore correct option is (A).
Note:We should note that diborane can be used as a rocket propellant. The complete combustion of diborane is strongly exothermic. But we should also know that this shows the similarity with carbon monoxide and doesn’t burn completely.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




