
Azo dye is prepared by the coupling of phenol and?
A. Diazonium chloride
B. o-nitroaniline
C. Benzoic acid
D. Chlorobenzene
Answer
241.5k+ views
Hint: Azo dye is an organic compound formed by a diazo coupling reaction. Azo dye has an azo functional group i.e., where this azo bond is generally attached to the aromatic rings. This reaction is an electrophilic substitution reaction where phenol act as a nucleophile.
Complete Step by Step Solution:
From the option, we need to find the one which is electrophilic so that our nucleophile i.e., phenol can attach to it and also can form an azo bond to prepare the azo dye. In the option, only diazonium chloride is the compound with a diazo bond i.e., and can directly form an azo bond with nucleophiles.
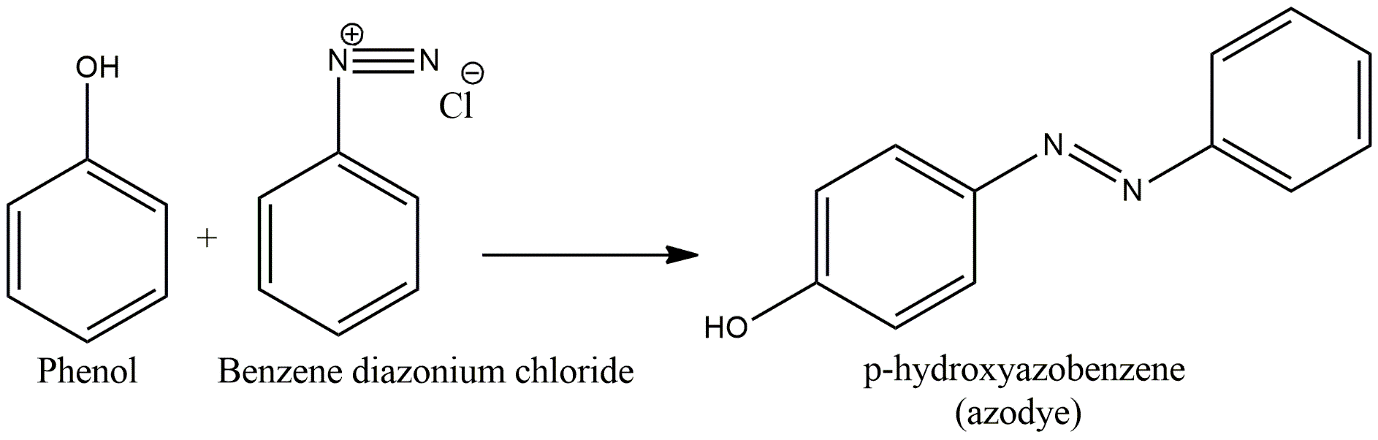
Image: Azo Dye preparation by coupling of phenol and diazonium chloride salt
So, option A is correct.
Additional Information: Options B, C and D cannot form azo dye because first, they cannot make an azo bond and second, they cannot attract nucleophiles better than diazonium chloride. Because in the o-nitroaniline nitro functional group is present which is an electron-withdrawing character and reduces the nucleophilic character of an aromatic ring. In benzoic acid again, the carboxylic acid functional group is taking part in the delocalisation of electrons of benzene and hence reduces the nucleophilic properties. In chlorobenzene -I effect of chlorine decreases the electron density on the benzene ring and reduces its nucleophilic character. Azo dyes are widely used in the colouring of paper, textiles and printing industries.
Note: Azo bond is \[ - N = N - \] and diazo bond is \[ - \mathop N\limits^ \oplus \equiv N\]. Electrophiles are those which have more electron density and attack nucleophiles to give a nucleophilic reaction and nucleophiles have less electron density and attach with electrophiles to give an electrophilic reaction.
Complete Step by Step Solution:
From the option, we need to find the one which is electrophilic so that our nucleophile i.e., phenol can attach to it and also can form an azo bond to prepare the azo dye. In the option, only diazonium chloride is the compound with a diazo bond i.e., and can directly form an azo bond with nucleophiles.

Image: Azo Dye preparation by coupling of phenol and diazonium chloride salt
So, option A is correct.
Additional Information: Options B, C and D cannot form azo dye because first, they cannot make an azo bond and second, they cannot attract nucleophiles better than diazonium chloride. Because in the o-nitroaniline nitro functional group is present which is an electron-withdrawing character and reduces the nucleophilic character of an aromatic ring. In benzoic acid again, the carboxylic acid functional group is taking part in the delocalisation of electrons of benzene and hence reduces the nucleophilic properties. In chlorobenzene -I effect of chlorine decreases the electron density on the benzene ring and reduces its nucleophilic character. Azo dyes are widely used in the colouring of paper, textiles and printing industries.
Note: Azo bond is \[ - N = N - \] and diazo bond is \[ - \mathop N\limits^ \oplus \equiv N\]. Electrophiles are those which have more electron density and attack nucleophiles to give a nucleophilic reaction and nucleophiles have less electron density and attach with electrophiles to give an electrophilic reaction.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
CBSE Class 12 Chemistry Question Paper 2026 PDF Download (All Sets) with Answer Key

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More




