
Acetonitrile is:
(a) \[{C_2}{H_5}CN\]
(b) \[C{H_3}CN\]
(c) \[C{H_3}COCN\]
(d) \[{C_6}{H_5}C{H_2}CN\]
Answer
233.1k+ views
Hint: 2-propenenitrile (\[C{H_2} = CH - CN\]) is an organic compound, in which the alkene group is directly attached to the carbon atom of the cyanide group (\[ - CN\]). Similarly, acetonitrile, the simplest organic nitriles also have a carbon-nitrogen triple bond. In acetonitrile, a methyl group is directly connected to the cyanide functional group.
Complete Step by Step Solution:
The nitriles are the class of organic compounds that contains the cyanide functional group (\[ - CN\]), in which a triple bond is present between carbon and nitrogen atom Image 1).
The nitriles are generally represented by the \[RCN\] chemical formula.

Image: A general structure of nitriles.
The nitriles are much less toxic than their inorganic analogues such as \[NaCN\] and \[KCN\].
Some compounds such as malonic malononitrile (\[NC{H_2}COOH\]), potassium ferricyanide (\[{K_3}[Fe{(CN)_6}]\]) and potassium ferrocyanide (\[{K_4}[Fe{(CN)_6}\]) are known as less toxic because these compounds do not have the tendency to liberate the cyanide group.
For the IUPAC nomenclature, the word nitrile is used after the name of the parent carbon chain. The carbon atom of the nitrile group is also counted as a part of the parent chain. For example, the IUPAC name of \[C{H_3}CN\] can be written as ethanenitrile and the carbon atom is also counted with the parent chain. The old name of \[C{H_3}CN\]is methyl cyanide and acetonitrile.
When the cyanide (\[ - CN\]) functional is group attached to the cycloalkane, The numbering starts from the carbon atom of cycloalkane which is directly attached to the cyanide group. The name of the cycloalkane is followed by the suffix -carbonitrile. The carbon atom of \[ - CN\]group is not involved in numbering.
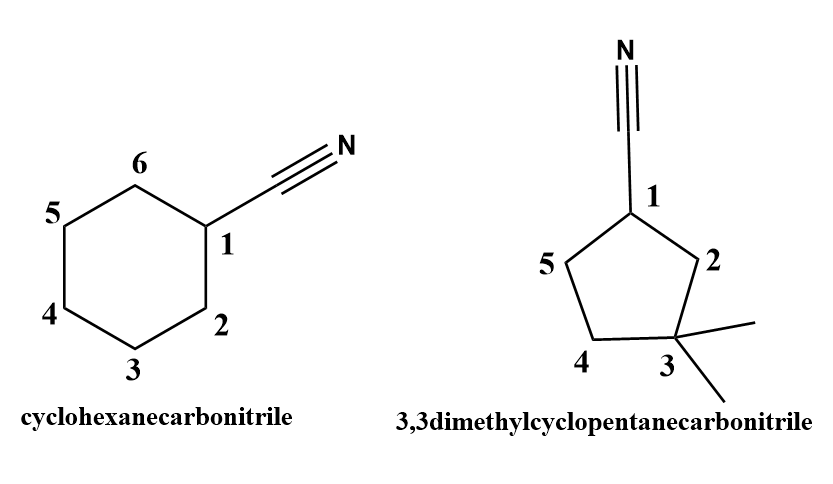
Image: Structure of cycloalkane containing \[ - CN\] functional group.
When a molecule contains a carboxyl group and nitrile functional group then the nitrile group is pronounced as the cyano group. The carbon atom of the cyano group is not counted as a part of the main chain. For example, 3-cyanopropanoic acid.
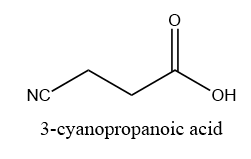
Image: Structure of a molecule having both cyano group and carboxylic group.
The above points indicate that the structure of acetonitrile will be \[C{H_3}CN\]. Therefore, option (b) will be the correct answer.
Note: Grignard reagent is one of the important compounds in organic chemistry. It is beneficial for the preparation of various organic compounds such as acetals, amides, amino compounds, etc. It is used for the manufacture of many compounds that are useful in the pharmaceutical field.
Complete Step by Step Solution:
The nitriles are the class of organic compounds that contains the cyanide functional group (\[ - CN\]), in which a triple bond is present between carbon and nitrogen atom Image 1).
The nitriles are generally represented by the \[RCN\] chemical formula.

Image: A general structure of nitriles.
The nitriles are much less toxic than their inorganic analogues such as \[NaCN\] and \[KCN\].
Some compounds such as malonic malononitrile (\[NC{H_2}COOH\]), potassium ferricyanide (\[{K_3}[Fe{(CN)_6}]\]) and potassium ferrocyanide (\[{K_4}[Fe{(CN)_6}\]) are known as less toxic because these compounds do not have the tendency to liberate the cyanide group.
For the IUPAC nomenclature, the word nitrile is used after the name of the parent carbon chain. The carbon atom of the nitrile group is also counted as a part of the parent chain. For example, the IUPAC name of \[C{H_3}CN\] can be written as ethanenitrile and the carbon atom is also counted with the parent chain. The old name of \[C{H_3}CN\]is methyl cyanide and acetonitrile.
When the cyanide (\[ - CN\]) functional is group attached to the cycloalkane, The numbering starts from the carbon atom of cycloalkane which is directly attached to the cyanide group. The name of the cycloalkane is followed by the suffix -carbonitrile. The carbon atom of \[ - CN\]group is not involved in numbering.

Image: Structure of cycloalkane containing \[ - CN\] functional group.
When a molecule contains a carboxyl group and nitrile functional group then the nitrile group is pronounced as the cyano group. The carbon atom of the cyano group is not counted as a part of the main chain. For example, 3-cyanopropanoic acid.

Image: Structure of a molecule having both cyano group and carboxylic group.
The above points indicate that the structure of acetonitrile will be \[C{H_3}CN\]. Therefore, option (b) will be the correct answer.
Note: Grignard reagent is one of the important compounds in organic chemistry. It is beneficial for the preparation of various organic compounds such as acetals, amides, amino compounds, etc. It is used for the manufacture of many compounds that are useful in the pharmaceutical field.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




