
A reaction of ethyl amine and acetic anhydride leads to the formation of:
(A) $C{{H}_{3}}NHCOC{{H}_{3}}$
(B) ${{C}_{2}}{{H}_{5}}CONHC{{H}_{3}}$
(C) $C{{H}_{3}}CONH{{C}_{2}}{{H}_{5}}$
(D) $C{{H}_{3}}-CH=NO{{C}_{2}}{{H}_{5}}$
Answer
241.2k+ views
Hint: A reaction of ethyl amine and acetic anhydride is an acetylation reaction. An acetylation reaction is the reaction in which an acetyl group is introduced into a compound. This reaction can occur by the addition of acetic anhydride or acetyl chloride. It is a nucleophilic substitution reaction. This reaction is also known as ethanoylation.
Complete Step by Step Answer:
The ethyl amine consists of an ethyl group attached to a $N{{H}_{2}}$ group. When the acetylation reaction of ethyl amine takes place by the addition of acetic anhydride, N-ethyl acetamide is formed. The chemical formula of N-ethyl acetamide is $C{{H}_{3}}CONH{{C}_{2}}{{H}_{5}}$. Nitrogen atoms of ethyl amine are acetylated in this reaction. In this reaction, one of the hydrogen atoms of the $N{{H}_{2}}$ group is replaced by an acetyl group.
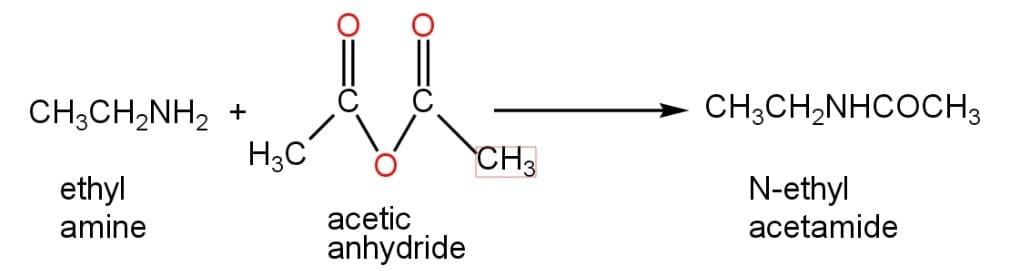
Correct Option: (C)$C{{H}_{3}}CONH{{C}_{2}}{{H}_{5}}$.
Additional Information: Many compounds like aspirin and proteins can be synthesised with the help of an acetylation reaction. These reactions play a crucial role in drug biotransformation, which is the acetylation reaction-aided processing and elimination of medicines by the body. The inverse reaction, which is the reaction in which the acetyl group is removed completely, is known as the deacetylation reaction.
Note: Acetylation is a modification that can significantly modify a protein's function by changing its hydrophobicity, solubility, and surface characteristics. These changes may have an impact on the protein's conformation and interactions with substrates, cofactors, and other macromolecules.
Complete Step by Step Answer:
The ethyl amine consists of an ethyl group attached to a $N{{H}_{2}}$ group. When the acetylation reaction of ethyl amine takes place by the addition of acetic anhydride, N-ethyl acetamide is formed. The chemical formula of N-ethyl acetamide is $C{{H}_{3}}CONH{{C}_{2}}{{H}_{5}}$. Nitrogen atoms of ethyl amine are acetylated in this reaction. In this reaction, one of the hydrogen atoms of the $N{{H}_{2}}$ group is replaced by an acetyl group.

Correct Option: (C)$C{{H}_{3}}CONH{{C}_{2}}{{H}_{5}}$.
Additional Information: Many compounds like aspirin and proteins can be synthesised with the help of an acetylation reaction. These reactions play a crucial role in drug biotransformation, which is the acetylation reaction-aided processing and elimination of medicines by the body. The inverse reaction, which is the reaction in which the acetyl group is removed completely, is known as the deacetylation reaction.
Note: Acetylation is a modification that can significantly modify a protein's function by changing its hydrophobicity, solubility, and surface characteristics. These changes may have an impact on the protein's conformation and interactions with substrates, cofactors, and other macromolecules.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
CBSE Class 12 Chemistry Question Paper 2026 PDF Download (All Sets) with Answer Key

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More




