




Introduction to Amino Acids and Peptides
Amino acids are the building blocks of proteins, but they also play a variety of functions in energy production and the development of a variety of other biomolecules such as hormones, neurotransmitters, and signalling molecules. Amino acid, peptide, and protein polymers choreograph and govern a wide range of physiologic and biochemical activities in humans. The catalogue of amino acids, peptides, and proteins found in diverse body fluids provides a target-rich environment for disease condition detection. This article provides an example of the types of questions that might be asked about this subject in the JEE exam.
Important Topics of Amino Acids and Peptides
Classes of Peptides
Types of Amino Acid
Protein Structure
Amino Acids
Proteins are made up of amino acids, which are the building components. When a person consumes protein-rich meals, the digestive system breaks the protein down into Amino Acids. The body then uses different combinations of amino acids to conduct physical functions. Because a healthy body can synthesise the other 11 amino acids on its own, they are rarely consumed through food.
R-CH(NH)2COOH is the formula for a simple amino acid (carboxyl group and amino group is present in it).
Alpha-Amino Acids
Alpha-amino acids are acids in which the NH2 group is located at a carbon atom next to the COOH group.
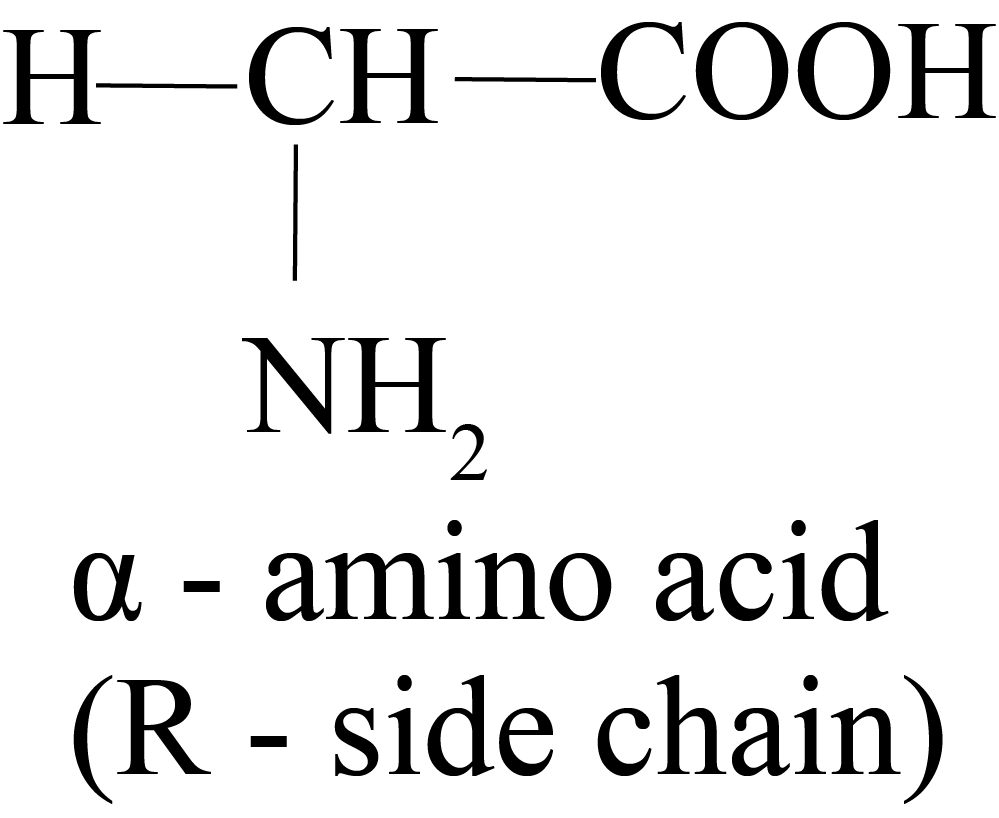
Proteins are made up of amino acids called alpha-amino acids. Because all amino acids include chiral alpha carbons, they all have stereoisomerism or the presence of D and L kinds of structures.
Alpha-amino acid occurs as a dipolar ion or Zwitterion due to proton transfer from carboxy to the amino group.
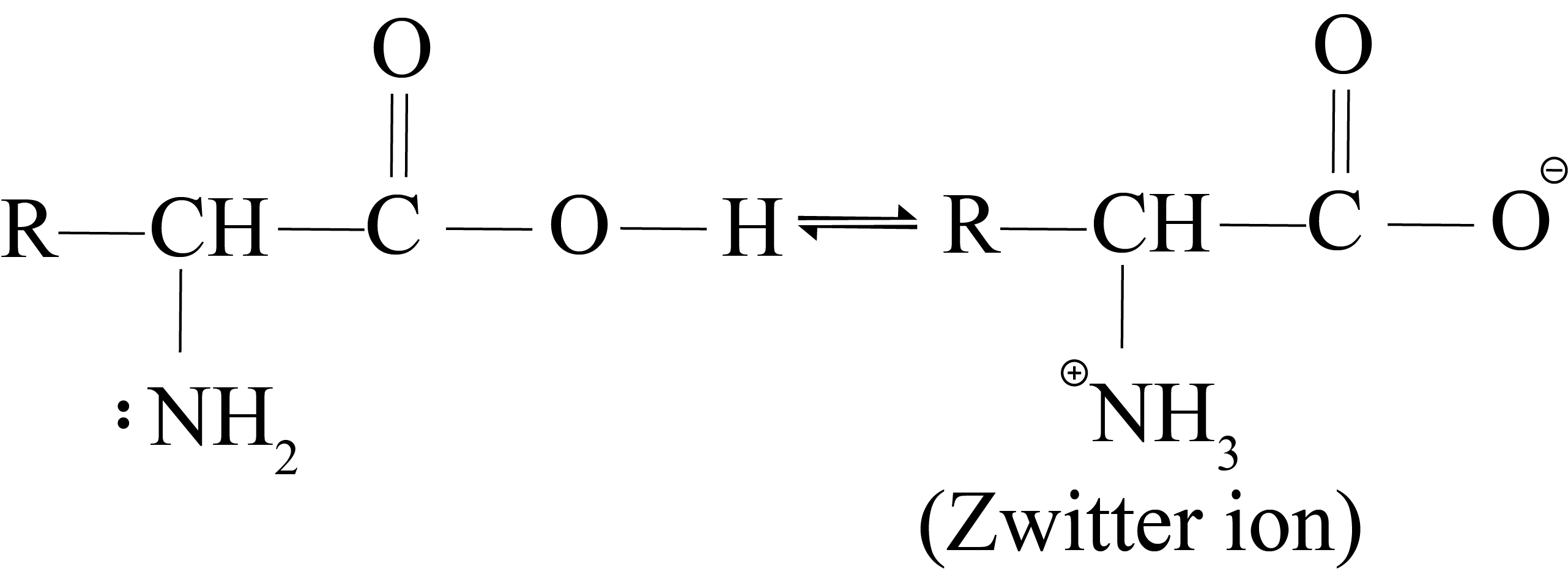
Isoelectric Point
Alpha-amino acids are high melting crystalline solids and somewhat soluble in water due to their zwitterion structure.
The carboxylate ion group acts as a base in acidic media and receives a proton. Under the influence of an electric field, alpha-amino acids exist as cations.
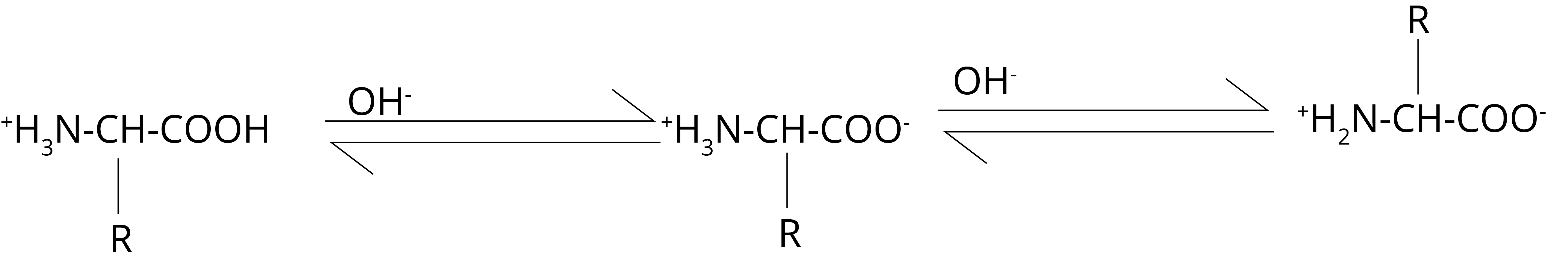
In an alkaline medium, NH3+ groups operate as acids, losing a proton in the process.
As a result, alpha-amino acids exist as anions (III) in the electric field and move towards the anode.
The concentrations of the cationic form (I) and the anionic form (III) become equal at some intermediate pH value.
As a result, there is no electric field migration. This pH is referred to as the isoelectric point.
Types of Amino Acid
There are two types of amino acids:
Essential
Non-essential amino acids
Essential Amino Acids
The amino acids that our bodies cannot produce and obtain from outside sources.
Lack of certain amino acids in the diet can lead to a variety of illnesses, including kwashiorkor (the disease in which water balance in the body is disturbed).
Out of all the amino acids, there are ten that are necessary.
Non-Essential Amino Acids
They are those that our bodies can manufacture.
The body can produce 10 amino acids out of a total of 20.
Although uncommon, it can cause neonates to grow insufficient weight. In adults, it can induce eczema, malaise, and memory loss.
General Properties of Amino Acids
Their melting and boiling points are very high.
Amino acids are crystalline solids that are white in colour.
Amino acids have a sweet, bitter, and tasteless flavour.
The majority of amino acids are water-soluble and insoluble in organic solvents.
Sources of Amino Acids
Amino acids are required for a number of biological and chemical processes in our bodies, including tissue growth and repair, enzyme creation and activity, food digestion, and molecule transportation. Because our bodies can only synthesise a certain amount of amino acids, we must receive the remaining amino acids, known as essential amino acids, from protein-rich foods in our daily diet. Broccoli, beans, beets, pumpkin, cabbage, almonds, dry fruits, chia seeds, oats, peas, carrots, cucumber, green leafy vegetables, onions, soybeans, whole grain, peanuts, legumes, lentils, and other plant-based foods are high in amino acids. Amino acids are abundant in apples, bananas, berries, figs, grapes, melons, oranges, papaya, pineapple, and pomegranates.
Functions of Essential Amino Acids
Phenylalanine aids in the maintenance of a healthy systema nervosum as well as memory enhancement.
Valine is a crucial component in increasing muscle development.
The amino acid threonine aids in the immune system's function.
Vitamin B3 and the serotonin hormone are both made from tryptophan. This serotonin hormone is important for hunger control, sleep regulation, and mood enhancement.
Isoleucine is essential for the creation of haemoglobin, increasing insulin synthesis in the pancreas, and carrying oxygen from the lungs to the various regions of the body.
Methionine is used in the treatment of kidney stones, the maintenance of healthy skin, and the control of harmful germs.
Protein synthesis and growth hormones are both aided by leucine.
Lysine is required for the creation and fixation of calcium in bones, as well as the synthesis of antibodies, hormones, and enzymes.
Histidine is involved in a variety of enzymatic activities as well as the formation of both red and white blood cells (erythrocytes) (leukocytes).
Functions of Non-Essential Amino Acids
Alanine helps our bodies remove toxins and aids in the creation of glucose and other amino acids.
Cysteine functions as an antioxidant, providing resistance to our bodies and inhibiting the growth of hair, nails, and other body parts.
Glutamine is required for the production of nucleic acids – DNA and RNA – and promotes healthy brain function.
Glycine aids in the proper development and function of cells, as well as the healing of wounds. It has the function of a neurotransmitter.
Glutamic acid is a neurotransmitter that plays an important role in the development and function of the human brain.
Arginine aids in the production of proteins and hormones, kidney cleansing, wound healing, and the maintenance of a healthy immune system.
Alanine helps our bodies remove toxins and is involved in the creation of glucose and other amino acids.
Cysteine is an antioxidant that gives our bodies resilience and prevents hair, nails, and other body parts from growing.
Glutamine is required for the production of nucleic acids, such as DNA and RNA, and it improves brain health.
Glycine aids in cell development, function, and wound healing. It has neurotransmitter properties.
Glutamic acid is a neurotransmitter that is essential for brain growth and function.
Arginine promotes protein and hormone synthesis, kidney cleansing, wound healing, and immune system function.
Deficiency of Amino Acids
Reduced immunity, digestive difficulties, depression, reproductive concerns, poorer mental alertness, delayed growth in children, and a variety of other health problems can all come from an amino acid deficit. Each essential amino acid has a particular purpose in the organism, and depletion symptoms vary correspondingly. The 9 essential amino acids are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
Peptides
The word peptide comes from the Greek word peptos, which means "digested." Peptides are amino acid chains that are short in length. Peptide bonds connect two or fifty amino acids in peptides. At the endpoints of the peptide chains, all peptides have an N-terminal and a C-terminal residue. In this scenario, cyclic peptides are an exception. N – terminal refers to the free amine group at the beginning of the polypeptide. The C – terminal refers to the free carboxyl group at the end of the polypeptide chain.
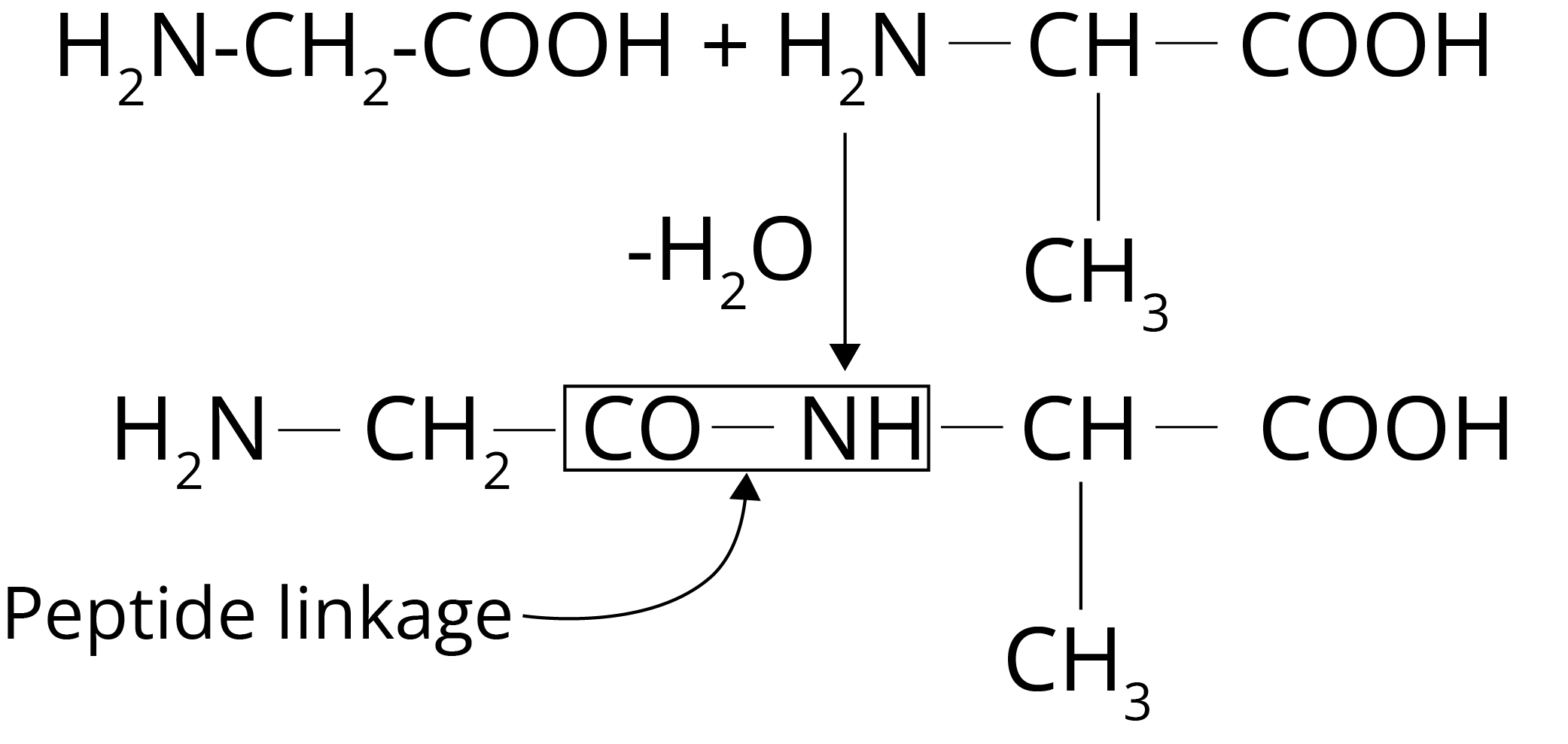
Polypeptide Formation
As previously stated, amino acids are the building elements of proteins, which play a critical part in practically all biological activities. As a result, in order to maintain a healthy and correct bodily function, we must incorporate all nine essential amino acids into our daily diet. There are a variety of pathological illnesses that can occur as a result of an amino acid shortage, including the following:
Diarrhoea, Anaemia, Depression, Edema, Hypoglycemia, Insomnia, Headache, weakness, irritability, and exhaustion.
Types or Classes of Peptides
Peptides are categorised based on their origins and functions. A few of its primary classes have been described in the following sections:
Ribosomal Peptides - Proteolysis is used to break down ribosomal peptides. They act as hormones in higher organisms. Antimicrobial peptides, tachykinin peptides, vasoactive intestinal peptides, pancreatic polypeptides, and more forms exist.
Peptones - They are generated when milk and animal proteins are broken down. They're used to cultivate fungus and bacteria for a variety of reasons.
Milk Peptides - They are generated when milk protein is digested. They can also arise as a result of milk fermentation.
Dipeptides - They are peptides made up of two amino acids linked by a single peptide bond. Carnosine, anserine, and other amino acids are examples.
Tripeptides - These peptides are made up of three amino acids linked together by two peptide bonds. Glutathione, ophthalmic acid, and other antioxidants are examples.
Oligopeptides - These peptides are made up of more than two but fewer than 20 amino acids linked together by peptide bonds. Netropsin, amanitin, and other similar proteins are examples.
Difference Between Peptide and Protein
Functions of Peptides
Peptides are the most basic chemical molecules found in cells. They play a variety of critical roles in the cell. They play important functions in a variety of biological events in the human body. The following are a few of its features:
They are necessary for muscular development.
GHRP 6 (Growth hormone releasing peptide 6) is important for our body's development.
Neuropeptides are chemical messengers utilised by neurons to interact with one another.
Peptides, such as peptide hormones, behave like hormones.
A peptide called an opioidergic agent works by directly modulating the opioid neuropeptide systems in the brain.
Copper peptides help to achieve beautiful skin and provide protection from the sun.
Some peptides are also used to treat hunger.
They're also used to treat illnesses.
Solved Examples from the Chapter
Example 1: The observation of “Rhumann’s purple” is a confirmatory test for the presence of:
Starch
Reducing sugar
Cupric ion
Protein
Solution:
Ninhydrin is the purple of Rhumann. It is used to determine if an alpha-amino acid or a carboxylic acid is present. Ruhemann's purple is a deep blue or purple hue created by interacting with free amines (proteins). As a result, the presence of "Rhumann's purple" is a confirming test for protein.
As a result, option (D) is the correct answer.
Key point to remember: 'Ruhemann's purple' is created when ninhydrin interacts with the alpha-amino group of primary amino acids.
Example 2: Biuret test is not given by:
Proteins
Carbohydrates
Polypeptides
Urea
Solution:
An amide linkage provides the biuret test. Proteins, polypeptides, and urea all contain it. Carbohydrates do not include it.
As a result, option (B) is the correct answer.
Key point to remember: The biuret test, commonly known as Piotrowski's test, is a chemical procedure for identifying peptide bonds.
Solved Questions from the Previous Years’ Question Papers
Question 1: Which of the following assertions is the most accurate?
Except for lysine, all amino acids are optically active.
Optical activity is present in all amino acids.
Except for glycine, all amino acids are optically active.
Except for glutamic acids, all amino acids are optically active.
Solution:
At the alpha carbon, glycine has two hydrogen atoms. Except for glycine, all amino acids are optically active. As a result, option (C) is the correct answer.
Trick: Remember the structure of amino acids.
Question 2: The functional group, which is found in an amino acid is:
COOH group
NH2 group
CH3 group
both (1) and (2)
Solution:
The answer is (D). COOH and NH3 are the functional groups present in amino acids (amine group), so each amino acid contains a core carbon with an H, R group (which changes depending on the amino acid), carboxylic group, and an amine group. A single (aliphatic) carbon connects these groupings. The alpha position is the carbon immediately connected to a carboxyl group, hence all amino acids in proteins are alpha-amino acids.
Trick: Remember the structure of amino acids.
Question 3: Insulin is:
An amino acid
Protein
A carbohydrate
A lipid
Solution:
Insulin is a hormone produced by the pancreas, which is located beneath the stomach. It enables your body to use glucose as a source of energy. Glucose is found in humans in the form of sugar. Insulin causes cells all over your body to absorb glucose and utilise it for energy when it enters your bloodstream. Insulin also aids in the regulation of blood glucose levels. Insulin is a protein made up of two chains of 51 amino acids.
Hence option (B) is correct.
Trick: Insulin by chemical nature is protein.
Practice Questions
Question 1: Peptides are:
Esters
Salts
Amides
Ketones
Answer: (C) Amides
Question 2: The proteins which are insoluble in water are
Fibrous proteins
Globular proteins
Both A and B
None of these
Answer: (A) Fibrous proteins
Conclusion
Thus, the study reveals that the proteins are made up of amino acids, which are the building components. When a person consumes protein-rich meals, the digestive system breaks the protein down into amino acids.
Peptides are amino acid chains that are short in length. Peptide bonds connect two or fifty amino acids in peptides. Hence, this is important not only for competitive exams like JEE or NEET but also for a better understanding of the topics being covered.
FAQs on JEE Important Chapter - Amino Acids and Peptides
1. What are the 4 types of amino acids?
Amino acids are normally made up of a carbon atom, a hydrogen atom, a carboxyl group, an amino group, and a variable group from a structural standpoint. Amino acids are divided into four types based on their charge: nonpolar, polar, negatively charged, and positively charged.
2. How many types of peptides are there?
Peptides are often classified into three groups: According to the number of amino acids in the chain. Oligopeptides are short chains of amino acids, whereas polypeptides are larger chains of 20 to 50 amino acids. Two, three, and four amino acids make up dipeptides, tripeptides, and tetrapeptides, respectively.
3. How are peptides formed?
On a molecular level, a peptide bond is created through a dehydration synthesis or reaction. This is also known as a condensation reaction, and it happens most commonly between amino acids.























