
Easy Preparation for JEE Advanced with Class 12 Chemistry Chapter 5 Surface Chemistry Important Questions
To secure a strong rank in the JEE Advanced exam, mastering core concepts is key. Given the significance of Chemistry in JEE Advanced preparation, diligent reading and problem-solving across all chapters are vital. Particularly, Surface Chemistry, a pivotal chapter in JEE Advanced, demands attention. Vedantu's subject experts have curated a set of essential questions specifically for Surface Chemistry Class 12 for JEE Advanced. These questions serve as a valuable resource, allowing students to reinforce their understanding and readiness for the exam. With this targeted practice, students can navigate the intricacies of Surface Chemistry confidently and enhance their overall performance in JEE Advanced.
Category: | JEE Advanced Important Questions |
Content-Type: | Text, Images, Videos and PDF |
Exam: | JEE Advanced |
Chapter Name: | Surface Chemistry |
Academic Session: | 2026 |
Medium: | English Medium |
Subject: | Chemistry |
Available Material: | Chapter-wise Important Questions with PDF |
Solving the important questions from the worksheets will make sure that students are able to grasp the concept of Surface Chemistry and answer questions in the JEE Advanced with ease. Download JEE Advanced Surface Chemistry Important Questions right now and prepare well for your exam.
Access JEE Advanced Important Questions Chemistry Surface Chemistry
Single Correct Type
Which of the following factors contributes to the additional stability of the lyophilic colloid?
(A) Colour
(B) Charge
(C) Hydration
(D) Tyndall effect
Critical temperatures of $S{O_2},{N_2},N{H_3}$ and $C{H_4}$ are 430 k, and 126 kk. 460k and 356k. In descending order, the volume of these gases adsorbed per gram of charcoal.
JEE Adv. - 2016
(A) $S{O_2} > N{H_3} > C{H_4} > {N_2}$
(B) $S{O_2} < N{H_3} < C{H_4} < {N_2}$
(C) $S{O_2} > N{H_3} = C{H_4} > {N_2}$
(D) $S{O_2} = N{H_3} = C{H_4} > {N_2}$
Gold number of four protective colloids P, Q, R, and S are 0.5, 0.01, 0.1, and 0.005, respectively, Arrange them in the correct order of their protective power
(A) $D < A < C < B$
(B) $D < B < C < A$
(C) $A < D < C < B$
(D) $C < A < D < B$
$\dfrac{x}{m}=K{{C}^{1/n}}$ where C= concentration of the solution, x=weight of the adsorbed solute; is equal to the weight of adsorbent, the equation formed could be (A) A hyperbola
(B) A parabola
(C) A straight line with a positive slope
(D) A straight line with a negative slope
Above this, it is called the temperature at which the gas constant cannot be liquefied even when high pressure is applied.
(A) Boiling point
(B) freezing point
(C) Critical temperature
(D) Boyle’s temperature
Multiple Correct Answers
To coagulate 100 ml of arsenious sulfide solution, 5ml of 1M NaCl is required. Calculate the flocculation value
(A) Number of electrolytes NaCl required to Coagulate 100 ml is 5
(B) Number of electrolyte NaCl required to Coagulate 100 ml is 50
(C) Flocculating value = 5 molL-1
(D) Flocculating value = 0.05 molL-1
Which can absorb maximum amount of ${{H}_{2}}$
(A) A platinum black
(B) Powdered palladium
(C) A platinum rod
(D) Nickel sphere
One gram of charcoal adsorbs 100ml of 0.5M to form a monolayer; the molarity of acetic acid is reduced to 0.49M. Calculate the surface area of the charcoal adsorbed by each molecule of acetic acid (Surface area of charcoal is.
JEE Adv. - 2015
(A) Moles of acetic acid before the adsorption 0.05.
(B) Number of moles of acetic acid after the adsorption 0.049.
(C) Number of molecules of acetic acid adsorbed $6.023 \times {10^{20}}$
(D) Surface area of the charcoal occupied by each acetic acid molecule $5 \times {10^{ - 19}}{m^2}$
Choose the correct reasons for the stability of the lyophobic colloidal particle.
(A) Preferential adsorption of ions of their surface from the solution.
(B) Preferential adsorption of solvent on their surface forms the solution.
(C) Attraction between different particles having opposite charges on their surface.
(D) Potential difference between the fixed layer and the diffused layer of opposite charge around the colloidal particles.
The hydrophobic end of sodium lauryl sulfate
(A) ${{C}_{17}}{{H}_{35}}$
(B) ${{C}_{17}}{{H}_{33}}$
(C) ${{C}_{12}}{{H}_{25}}$
(D) $-OSO_{3}^{-}$
In Langmuir adsorption isotherm, what is the slope and Y-intercept
(A) $\dfrac{x}{m} = \dfrac{{aP}}{{1 + bP}}$
(B) $\dfrac{{{x_1}}}{{{x_2}}} = {\left( {\dfrac{{{P_1}}}{{{P_2}}}} \right)^{1/n}}$
(C) $\dfrac{P}{{x/m}} = \dfrac{1}{a} + \left( {\dfrac{b}{a}} \right)P$
(D) $\dfrac{P}{{x/m}} = \left( {\dfrac{b}{a}} \right)P + \dfrac{1}{a}$
The qualitative sketches I, II, and III given below show the variation of surface tension with a molar concentration of three different aqueous solutions of $KCl,C{H_{3}}OH\;and\;C{H_3}{(C{H_2})_{11}}OS{O_3}^{ - }N{a^ + }$at room temperature. The correct assignment of the sketches is:
JEE Adv. - 2018
(A) | I | KCl | II | $\mathrm{CH}_{3} \mathrm{OH}$ | III | $\mathrm{CH}_{3}\left(\mathrm{CH}_{2}\right)_{11} \mathrm{OSO}_{3}{ }^{-} \mathrm{Na}^{+}$ |
(B) | I | $\mathrm{CH}_{3}\left(\mathrm{CH}_{2}\right)_{11} \mathrm{OSO}_{3}{ }^{-} \mathrm{Na}^{+}$ | II | $\mathrm{CH}_{3} \mathrm{OH}$ | III | KCl |
(C) | I | KCl | II | $\mathrm{CH}_{3}\left(\mathrm{CH}_{2}\right)_{11} \mathrm{OSO}_{3}{ }^{-} \mathrm{Na}^{+}$ | III | $\mathrm{CH}_{3} \mathrm{OH}$ |
(D) | I | $\mathrm{CH}_{3} \mathrm{OH}$ | II | KCl | III | KCl |
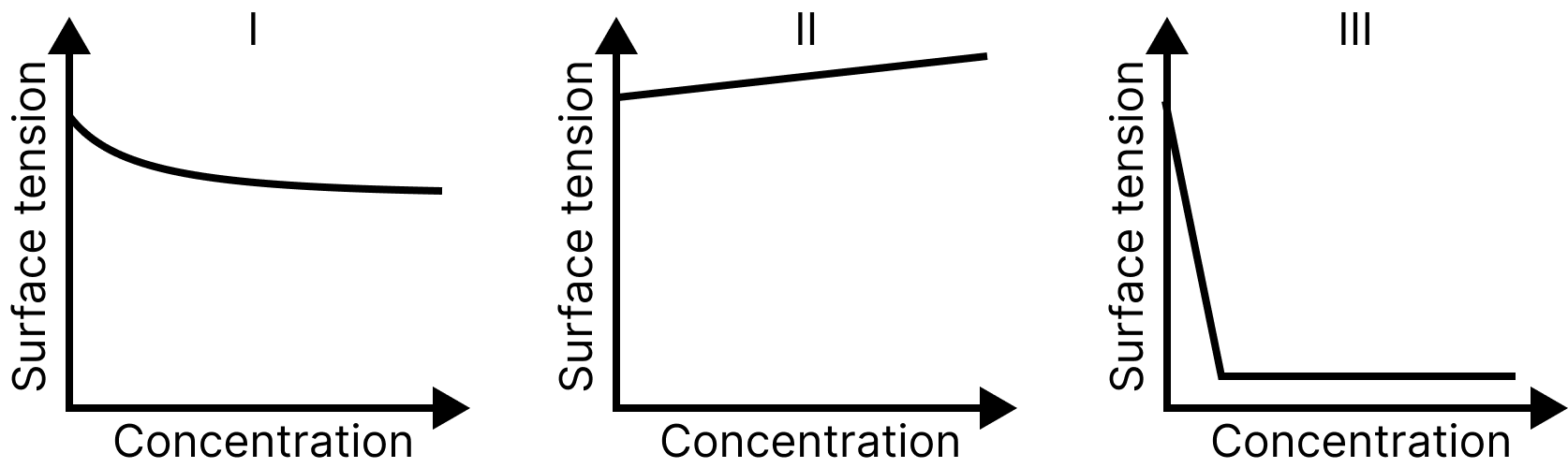
Surface Tension and Concentration
JEE Adv. - 2018
Which of the following statements are correct?
(A) Mixing two oppositely charged sols neutralizes their charges and stabilizes the colloid.
(B) Presence of equal and similar charges on colloidal particles provides stability to the colloids.
(C) Any amount of dispersed liquid can be added to emulsion without destabilizing it.
(D) Brownian movement stabilizes sols.
The given graph/data I, II, III, and IV represent general trends observed for different physisorption and chemisorption processes under mild temperature and pressure conditions. Which of the following choice(s) about I, II, III, and IV is (are) correct?
JEE Adv. - 2015
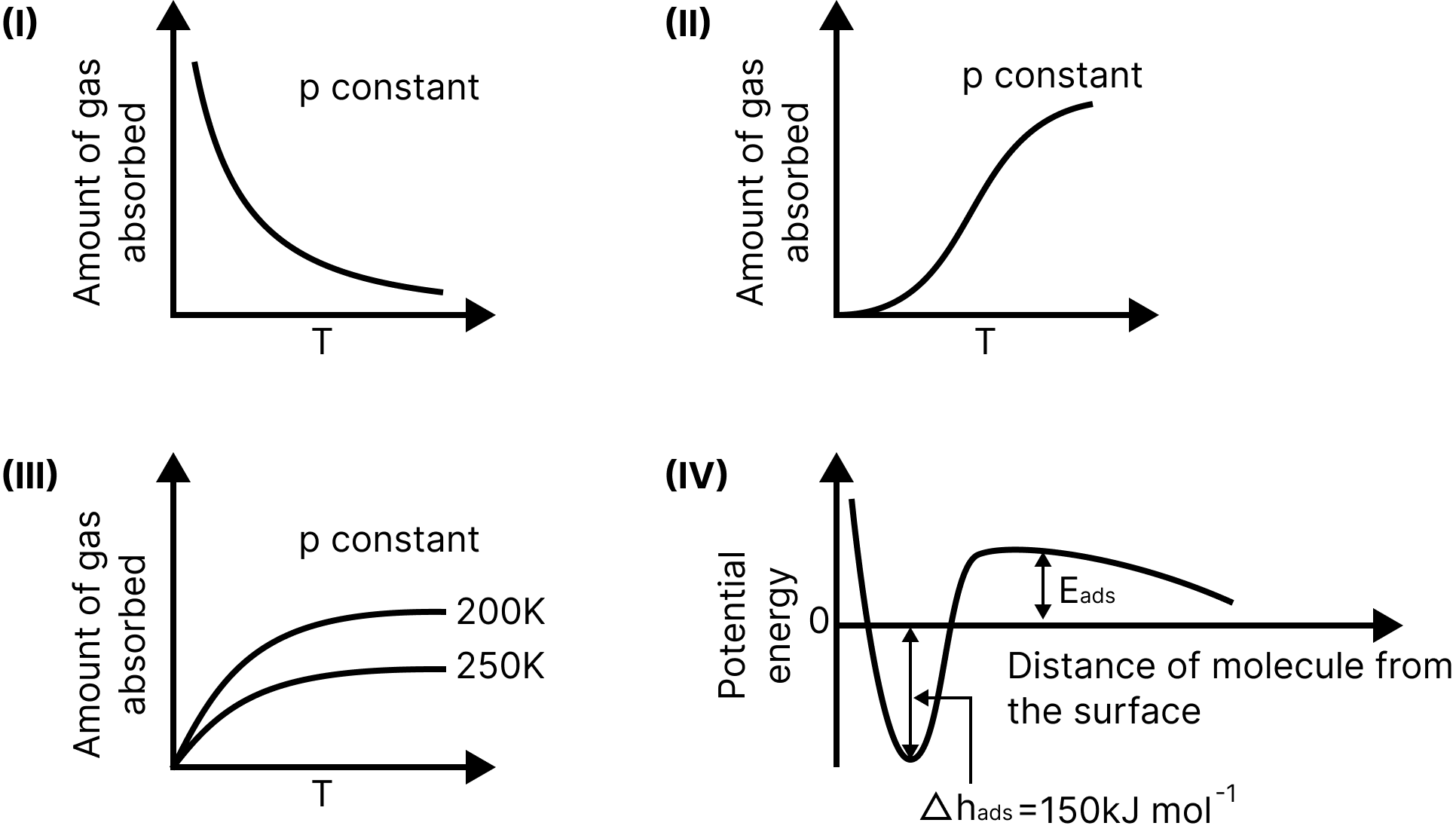
Graph (I, II, III, IV)
(A) I is physisorption and II is chemisorption
(B) I is physisorption and III is chemisorption
(C) IV is chemisorption and II is chemisorption
(D) IV is chemisorption and III is chemisorption
Favourable conditions for physical adsorption are:
(A) Low temperature
(B) High temperature
(C) Low pressure
(D) High pressure
The correct statement(s) about the adsorption of a gas on a solid surface is (are)
JEE Adv. - 2011
(A) Adsorption is always exothermic.
(B) Physisorption may transform into chemisorption at high temperatures.
(C) Physisorption increases with increasing temperature, but chemisorption decreases with increasing temperature.
(D) Chemisorption is more exothermic than physisorption; however, it is very slow due to higher activation energy.
The catalyst used in the dehydration of ethyl alcohol to ether is
(A) $A{{l}_{2}}{{O}_{3}}$
(B) $S{{b}_{2}}{{O}_{3}}$
(C) $A{{s}_{2}}{{O}_{3}}$
(D) $Cu$
The coagulation of 100ml gold sol is completely prevented by adding 0.25g of starch to it before adding 10 ml of 10% NaCl solution. Calculate the gold number of starch.
(A) 10% NaCl solution is added
(B) weight of starch required =0.25g
(C) weight of starch required =0.025g
(D) Gold number of starch =25
The correct statement(s) about surface properties is (are):
JEE Adv - 2017
(A) Cloud is an emulsion type of colloid in which liquid is dispersed phase and gas is dispersion medium.
(B) Adsorption is accompanied by a decrease in enthalpy and a decrease in entropy of the system.
(C) The critical temperature of ethane and nitrogen are 563 K and 126 K, respectively. The ethane adsorption will be more than nitrogen on the same amount of activated charcoal at a given temperature.
(D) Brownian motion of colloidal particles does not depend on the size of the particles but depends on the viscosity of the solution.
A catalyst is a substance which
(A) Increases the equilibrium concentration of the product
(B) Changes the equilibrium constant of the reaction
(C) Shortens the time to reach equilibrium
(D) Supplies energy to the reaction
Integer Type Questions
Per 2 gm of charcoal, a gas is adsorbed by 0.1g and 0.2g at 10 torrs and 80 torr pressure, respectively, Calculate the n value in Freundlich adsorption isotherm.
If 1 ml of 10% NaCl solution is added, 0.025 g of starch sol is required to prevent coagulation of 10 ml of gold sol. How many golds is the starch sol?
Finely divided catalysts have a larger surface area and greater catalytic activity than compact solids. If a total surface area of 6291456 cm2 is required for adsorption of gaseous reactants in a catalytic reaction, how many cubes of exactly 1 cm length need to be divided? Given: After each split, a new cube of the same size will be created.
JEE Adv. - 2019
The amount of ${N_2}$ required to form a monomolecular layer on the surface of the iron catalyst with NTP is 8.15 ml / g of adsorbent. If each nitrogen molecule occupies $16 \times {10^{ - 22}}{m^2}$, what is the surface area of 100 g of adsorbent?
The coagulation values of the electrolytes $AlC{l_3}$ and NaCl in the $A{s_2}{S_3}$ sol is 0.093 and 52, respectively. How often does $AlC{l_3}$ coagulate more than NaCl?
Solutions
(A)
Hydration helps improve the stability of lyophilized colloids, as positivity means preferring liquids.
(A)
The extent of adsorption is directly proportional to critical temperature, order of extent of adsorption of the given gases is: $S{O_2} > N{H_3} > C{H_4} > {N_2}$.
(B)
The lesser the value of the gold number, the higher will be the protective power.
(C)
The plot of this equation is a straight line, as shown in the following curve.
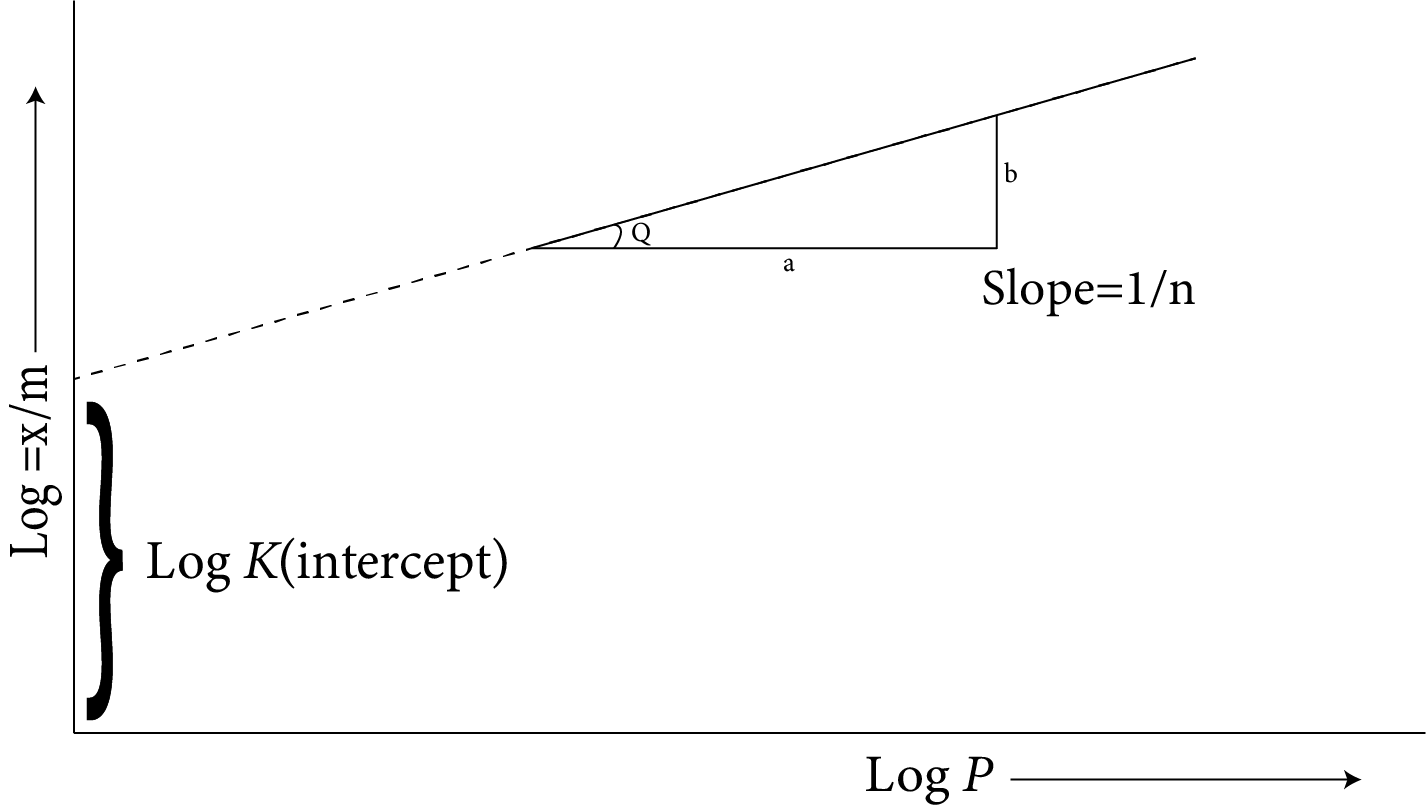
(A)
The temperature at which the gas cannot be liquefied beyond that is called the critical temperature ${T_C}$ The critical temperature of a substance is above the temperature at which the vapor of the substance cannot be liquefied no matter how much pressure is applied.
(A, B and D)
Number of millimoles of electrolyte NaCl required to Coagulate 100ml of sol$ = 1 \times 5 = 5$
Number of millimoles of electrolyte required to Coagulate 1000ml of sol$ = 5 \times \dfrac{{1000}}{{100}} = 50$
The minimum number of moles of electrolyte per liter required to cause precipitation is called the Flocculation value. Flocculating value =50 millimoles= 0.05 molL-1
B
Since the colloidal solution of palladium has the largest surface area, the colloidal solution of palladium can adsorb the maximum amount of hydrogen gas. The larger the surface area of the catalyst, the more hydrogen will be adsorbed.
(A, B, C, and D)
Number of moles of acetic acid before the adsorption= $0.5 \times \dfrac{{100}}{{1000}} = 0.05$
Number of moles of acetic acid after the adsorption= $0.49 \times \dfrac{{100}}{{1000}} = 0.049.$
Number of molecules of acetic acid adsorbed= $0.001 \times 6.023 \times {10^{23}} = 6.023 \times {10^{20}}$
The surface area of the charcoal occupied by each acetic acid molecule
= $\dfrac{{3.01 \times {{10}^2}}}{{6.023 \times {{10}^{20}}}} = 5 \times {10^{ - 19}}{m^2}.$
(A and D)
Due to the preferential adsorption of common ions, a layer of oppositely charged particles around the colloidal particles, due to repulsion rather than attraction, reduces the potential energy of the entire system.
(D)
Sodium lauryl sulfate is an amphipathic molecule that exhibits the presence of both hydrophobic and hydrophilic chains. The hydrophilic head is the part that contains $-OSO_{3}^{-}$. The hydrophobic tail is an alkyl chain containing 12 carbon atoms and 25 hydrogen atoms.
Sodium lauryl sulfate is used as a cleaning detergent and degreasing agent. The sulfonic acid and dodecanol are esterified to produce the resulting compound.
(A, B, C and D)
A plot $\left( {\dfrac{p}{{x/m}}} \right)$ versus P is a straight line with Y-intercept equal to 1/a and slope equal to b/a.
(D)
Impurities have a great effect on surface tension. Impurities that tend to concentrate on the surface of liquids have been observed to reduce surface tension compared to their bulk.
Substances such as detergents and soaps $[{(CH)_{3}}{({(CH)_{2}})_{11}}S{O_3}^ - {(Na)^ + }]$ decreases the surface tension sharply. Those like alcohol (e.g.,$C{H_3}OH,{C_2}{H_{5}}OH$ )lower the surface tension slightly.
Inorganic impurities present in the bulk of a liquid such as KCl tend to increase the surface tension of water.
(B and D)
The presence of similar charges on colloidal particles gives the colloid stability. This prevents the particles from colliding when they approach each other due to the repulsive force between similarly charged particles.
(A and C)
Figure I shows the adsorbent's physical adsorption by a weak van der Waals force. Increasing the temperature increases the kinetic energy of the adsorbed particles, which increases the desorption rate, reducing the adsorption.
Graph II shows chemisorption because the amount of chemisorption increases as the temperature rises.
Graph-IV represents chemisorption because graphs are characteristic of binding forces.
Gravity is dominant until a bond is formed. The repulsive force becomes dominant the next time you try to reduce the distance.
(A and D)
High temperature is not a suitable condition for physisorption. Physisorption is an exothermic process. Use at low temperatures is preferred. This corresponds to Le Chatelier's principle. The degree of adsorption decreases with increasing temperature.
(A, B and D)
Adsorption is always exothermic. Physisorption may transform into chemisorption at high temperatures.
Chemisorption is more exothermic than physisorption; however, it is very slow due to the higher activation energy. Physisorption decreases with an increase in temperature. But chemisorption first increases with an increase in temperature and then decreases. The effect is called activated adsorption.
(D)
The catalyst used to dehydrate ethyl alcohol to ether is Cu
(A, B and D)
10 ml of 10% NaCl solution is added to 100ml of gold sol. Thus, 1ml of 10% NaCl has been added to each 10ml of gold sol.
Weight of starch required for 100ml gold sol=0.25g=250mg and 10ml sol is 25mg.
The gold number of starch is 25.
(B and C)
(B) Adsorption is accompanied by a decrease in system enthalpy and a decrease in entropy. In the case of adsorption, both changes in enthalpy and changes in entropy are negative. The change in Gibbs free energy is also negative.
(C) The critical temperatures for ethane and nitrogen are 563K and 126K, respectively. Ethane adsorption is greater than nitrogen adsorption on the same amount of activated carbon at a particular temperature. The higher the critical temperature, the easier it is to liquefy the gas and the higher the degree of adsorption.
(C)
A catalyst is a substance that alters the rate of reaction and shortens the time to reach equilibrium.
3
$\dfrac{x}{m} = K.{P^{1/n}}$
Substituting the values,
$\dfrac{{0.1}}{{0.2}} = {\left( {\dfrac{{10}}{{80}}} \right)^{1/n}}(Or){\left( {\dfrac{1}{2}} \right)^1} = {\left( {\dfrac{1}{2}} \right)^{3/n}}$
Therefore, the value of n is 3.
25
By definition, the gold strength number is the amount of starch (mg) added to a 10 mL standard gold sol, which prevents gold from solidifying when 1 mL of a 10% NaCl solution is added. Therefore, the amount of straw is 0.25 g = 250 mg. Therefore, the gold number is 250.
20
Area = $6 \times {\left( {length} \right)^2}$
Length after n splits=$\dfrac{1}{{{2^n}}}$
Total surface area after n splits
$= {8^n} \times 6 \times length$
$= {8^n} \times 6 \times {\left( {\dfrac{1}{{{2^n}}}} \right)^2}$
$= {2^n} \times 6$
${2^n} \times 6 = 6291456$
${2^n} = 1048576$
$n \times 0.3010 = 6.0206$
$n = \dfrac{{6.0206}}{{0.3010}}$
$n = 20 splits$
35
${N_{2}} = 8.15 \times {10^{ - 3}}LofN{O_2}$
Moles of ${N_{2}} = \dfrac{{8.15 \times {{10}^{ - 3}}}}{{22.4}} = 0.3638 \times {10^{ - 3}}$
Molecules= $0.3638 \times {10^{ - 3}} \times 6.022 \times {10^{23}} = 2.19 \times {10^{20}}$
Surface area covered = $\left( {16 \times {{10}^{ - 29}}{m^2}} \right)\left( {2.19 \times {{10}^{20}}} \right)$
$= 35.072 \times {10^{ - 9}}{m^2}$
$= 0.35{m^2}/g$
559
The coagulating power of the electrolytes are inversely proportional to their coagulating values. Thus,
$\dfrac{{{\text{Coagulating power of AlC}}{{\text{l}}_{\text{3}}}}}{{{\text{Coagulating power of NaCl}}}}{\text{ = }}\dfrac{{{\text{52}}}}{{{\text{0}}{\text{.093}}}}{\text{ = 55}}9$
Thus, The coagulating power of $AlC{l_3}$ is 559 times more than that of NaCl.
Importance of JEE Advanced Surface Chemistry Important Questions
Surface Chemistry is an important chapter in the syllabus of JEE advanced. This topic deals with the events that take place on the surface of objects. Students will get to learn different terms such as Adsorption, Adsorbate, Adsorbent, desorption, absorption, and sorption. The chapter also discusses the difference between Adsorption and Absorption which can be helpful for students to understand how the processes work.
Students also get to learn about the mechanism of adsorption, its classification and the comparison between physical and chemical adsorption. They are introduced to the concept of the surface area of a solid adsorbent and the effect of pressure and temperature on it. Furthermore, they can read about Freundlich Adsorption Isotherm and factors that affect adsorption from solution. They can familiarise themselves with the idea of Chromatographic analysis and different types of catalysis such as heterogeneous, negative, and auto-catalysis.
By solving the Surface Chemistry JEE Advanced questions, students will get to learn different concepts in the chapter. They can solve numerical questions from the chapter and get enough practice to nail their JEE Advanced examination. By studying the question papers, students will have an idea about the exam pattern and scoring for different chapters.
Benefits of JEE Advanced Important Questions for the Chapter Surface Chemistry
Important questions help you focus on key concepts within the Surface Chemistry chapter.
You concentrate your efforts on the most relevant topics that are likely to appear in the JEE Advanced exam.
Practicing these questions helps you understand the pattern and format of questions that may be asked in JEE Advanced.
You become familiar with the types of questions and can develop effective strategies for solving them.
Identify and prioritize topics within Surface Chemistry that carry more weight in the JEE Advanced exam.
Focus more on areas that are frequently covered and are crucial for scoring well.
Solve important questions to apply theoretical knowledge to practical problem-solving scenarios.
Enhance your ability to think critically and apply concepts to different situations.
Practice with important questions helps you improve your time management skills.
Learn to allocate time efficiently to different sections and questions during the actual exam.
Regularly solving important questions allows you to assess your understanding of Surface Chemistry.
Identify weak areas and work on improving them to enhance overall proficiency.
Successfully solving important questions boosts your confidence.
You gain a sense of accomplishment, which is crucial for tackling challenging problems in the JEE Advanced exam.
Use important questions as a tool for comprehensive revision before the exam.
Cover a wide range of topics within Surface Chemistry, ensuring that you're well-prepared for any question that may arise.
Tailor your study plan based on the insights gained from solving important questions.
Devote more time to areas that require additional attention and practice.
Continuous practice with important questions enhances your problem-solving skills.
As a result, you become more adept at handling complex problems and improve your overall performance in the JEE Advanced exam.
Download Important Questions for Surface Chemistry Right Now
Do you want to get placed in JEE Advanced by scoring high marks in the examination? Well, download Surface Chemistry important questions with answers right now from Vedantu. Including these questions in your study material will give you the expertise and knowledge to perform well in such competitive examinations. Download the PDF files now and that too free of cost from Vedantu.
Conclusion
"Surface Chemistry Class 12 Important Questions for JEE Advanced Chemistry" from Vedantu are like a secret weapon for your exam prep. They're your guide to conquering this important chapter and acing your JEE Advanced Chemistry. By diving into these carefully crafted questions, you not only boost your chances of scoring well but also get a solid grip on the tricky topics. Think of it as your free ticket to a PDF full of wisdom, helping you confidently tackle the exam. So, why wait? Download, practice, and let these questions be your key to success!
FAQs on Surface Chemistry Class 12 Important Questions JEE Advanced Chemistry [Free PDF Download]
1. Why isn’t a colloidal system formed when a particular gas is mixed properly with another gas?
When the gases are mixed with one another, they tend to form a mixture which is homogeneous in nature. That is why the gases don’t form a colloidal system.
2. Are adsorbate particles attracted and retained on the adsorbent surface? Why?
Yes, the particles of an Adsorbate tend to get attracted to the adsorbent surface and are retained there. The imbalanced forces in the adsorbent surface are the reason why the adsorbate is attracted in the first place.
3. What does the term desorption mean?
Desorption can be defined as the process that releases any particular material through or from any surface.
4. Define the term sorption.
Sorption can be defined as the chemical and physical process that enables a substance to bind itself to another substance.


















