
JEE Advanced 2026 Solid State Revision Notes - Free PDF Download
Solids are different from fluids in various aspects. The molecules of a solid remain in a highly compacted state. The intermolecular attraction in the solids is the highest, causing no free flow of the molecules. This is why they have a specific volume and shape. Solid State is an important chapter covered in the JEE Advanced syllabus. To understand the various concepts of this chapter, go through the JEE Advanced Solid State revision notes prepared by the subject experts at Vedantu.
Category: | JEE Advanced Revision Notes |
Content-Type: | Text, Images, Videos and PDF |
Exam: | JEE Advanced |
Chapter Name: | Solid State |
Academic Session: | 2026 |
Medium: | English Medium |
Subject: | Chemistry |
Available Material: | Chapter-wise Revision Notes with PDF |
These Solid State JEE notes have been prepared as per the latest JEE syllabus to offer a better understanding of the advanced concepts. Students will be able to learn the theories and principles of Solid State here and resolve their doubts on their own.
JEE Advanced Revision Notes Chemistry Solid State
In crystalline solid, atoms, ions or molecules are arranged orderly in a three-dimensional manner due to strong binding force.
Due to this arrangement, crystalline solids acquire rigidity and definite geometric shape or crystalline structures.
Characteristics Properties of the Solids
They have definite mass, volume and shape.
Intermolecular distance is short.
Intermolecular forces are strong.
Their constituent particles (atom, molecules or ions) have fixed positions and can only oscillate about their mean position.
Their existence of a substance in more than one solid modification is called Polymorphism.
Anisotropy: Crystalline solids are anisotropic in nature, that is, some of their physical properties like electrical resistance or refractive index show different values when measured along with different directions in the same crystals. This arises from the different arrangements of particles in different directions.
Isotropy: Amorphous solids on the other hand are isotropic in nature, it is because there is no long-range order in them and arrangement is irregular along with all the directions. Therefore, the value of any physical property would be the same in any direction.
Annealing: An amorphous substance, on heating at a certain temperature, may attain crystalline nature while on cooling regains its amorphous nature, this phenomenon is called annealing.
Difference Between Crystalline and Amorphous Solids:
Property | Crystalline solids | Amorphous solids |
Shape | Definite characteristics, geometrical shape. | Irregular shape. |
Melting point | Melt at a sharp and characteristic temperature. | Gradually soften over a range of temperature. |
Cleavage property | When cut with a sharp-edged tool, they split into two pieces that are plain and smooth. | When cut with a Sharpe-edged tool, they cut into two pieces with an irregular surface. |
Heat of fusion | They have definite and characteristics value. | They do not have definite value. |
Anisotropy | Anisotropic in nature except, for cubic crystals. | Isotropic in nature. |
Nature | True solids | Pseudo solids or super cooled liquids |
Order in arrangement of constituent particles | Long range order | Only short-range order |
Law of crystallography | Follows | Does not follow |
X-ray diffraction bands | Forms | Does not form |
Cooling Curve | Cooling curve is not smooth 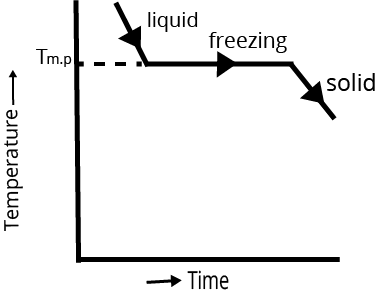
| Cooling curve is smooth 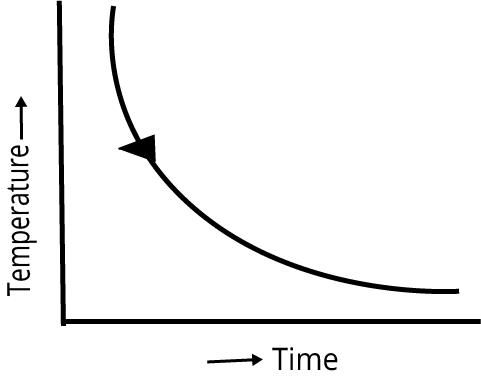
|
Example | NaCl, Diamond, MgO | Glass, Rubber, Plastic, Quartz glass |
Classification of Crystalline Solids:
A crystal is classified as ionic, covalent, metallic and molecular according to the nature of the building units, chemical bonding and the intermolecular forces in the crystal.
Different Types of Solids:
S.No | Types of Solid | Constituting Unit | Interparticle Force | Example | Physical Mode | Electrical Properties. |
1 | Molecular solids | |||||
i) polar-molecular solid. | Polar molecules | Dipole, dipole attraction forces | HCl, $S{O_2}$etc | Soft solid with low melting point | Insulator | |
ii) Non-polar molecular solids | Non -polar molecules | van der Waals’s force of attraction | \[BeC{l_{2,}},CC{l_4}\] | Soft solid with very low melting point | Insulator | |
iii) H-bonding solids | Molecules capable to from H-bond | H-bonds | $N{H_4}$,ice, glucose, etc | Harder than (i) and (ii) with low melting point | Insulator | |
2 | Covalent or network solids. | Atoms | Covalent bonds | Diamond, quartz, silica | Very hard melting point rigid | Insulator/ Conductor |
3 | Ionic solids | Cations and anions | Electrostatic force of attraction | NaCl, Zns | Hard rigid high m.pt | Conductor in fused state |
4 | Metallic solids | Metal atom (kernels embedded in sea of electrons) | Metallic bonds | Metal Cu, Ag, Mg, Fe | Hard but malleable and ductile | conductor |
Law of Crystallography:
The study of structure, geometry, and properties of crystals is called Crystallography.
Different Law of Crystallography is
Law of Constancy of Interfacial Angle: measured at the same temperature, a similar angle on crystals of the same substance remains constant regardless of the size and shape of the crystal.
Law of Constancy of Symmetry: The symmetry in all crystals of a particular species is constant though, i.e., the crystals of the same substance possess the same symmetry of element.
The Symmetry of Elements is:
Plane of symmetry
Axis of symmetry
Centre of symmetry
Law of Rational Indices: The law of rational indices states that the intercept of any face of crystal along the crystallographic axes is either equal to the unit intercept or some simple whole number multiples of them.
Unit Cell
The smallest portion of the crystal lattice which can be used as a repetitive unit in a three-dimension manner to get the entire crystal lattice is called a unit cell.
They are broadly divided into two categories, primitive and centred unit cells.
a. Primitive Unit Cell: when constituent particles are present only in the corner position of a unit cell, it is called a primitive unit cell
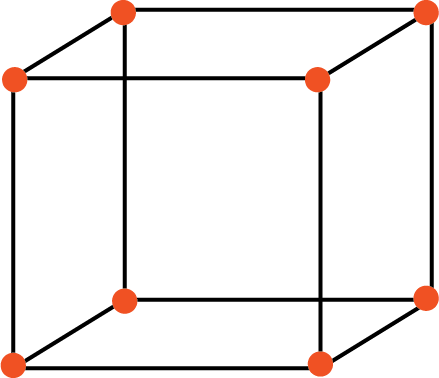
Primitive Unit Cell
b. Centred Unit Cell: When a unit cell contains one or more constituent particles present at a position other than corners in addition to those at corners, it is called a centred unit cell.
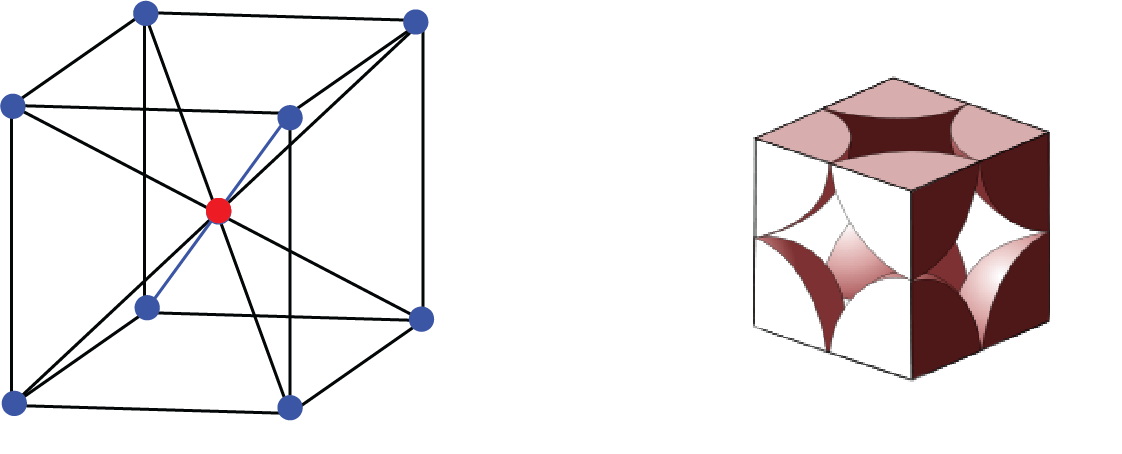
Centred Unit Cell
There are 3 Types:
Simple cubic cell
Body-centred unit cell
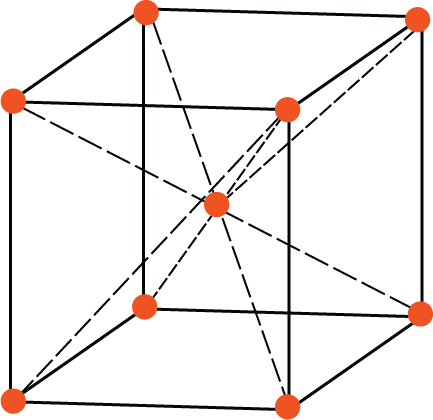
Body-Centred Unit Cell
Face-centred cell
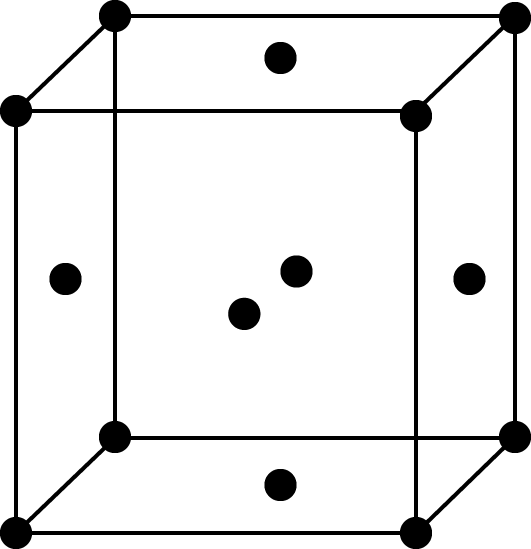
Face-Centred Cell
Bravais Lattices:
Bravais Lattices showed there are 14 different possible kinds of three-dimensional lattices.
These 14 Bravais lattices are grouped into seven crystal systems based on unit cell symmetry.
These Seven Crystal Systems are:
Crystal System | No. of Bravais Lattices | Possible Names of These Lattices |
Cubic | 3 | Simple cubic, fcc and bcc |
Tetragonal | 2 | Primitive body-centred face centre |
Orthorhombic | 4 | Primitive body-centred face centre and end centred |
Hexagonal | 1 | Primitive Hexagonal prism |
Trigonal | 1 | Rhombohedral (or) primitive |
Monoclinic | 2 | Primitive and end centred |
Triclinic | 1 | Triclinic primitive |
Seven Crystal Systems:
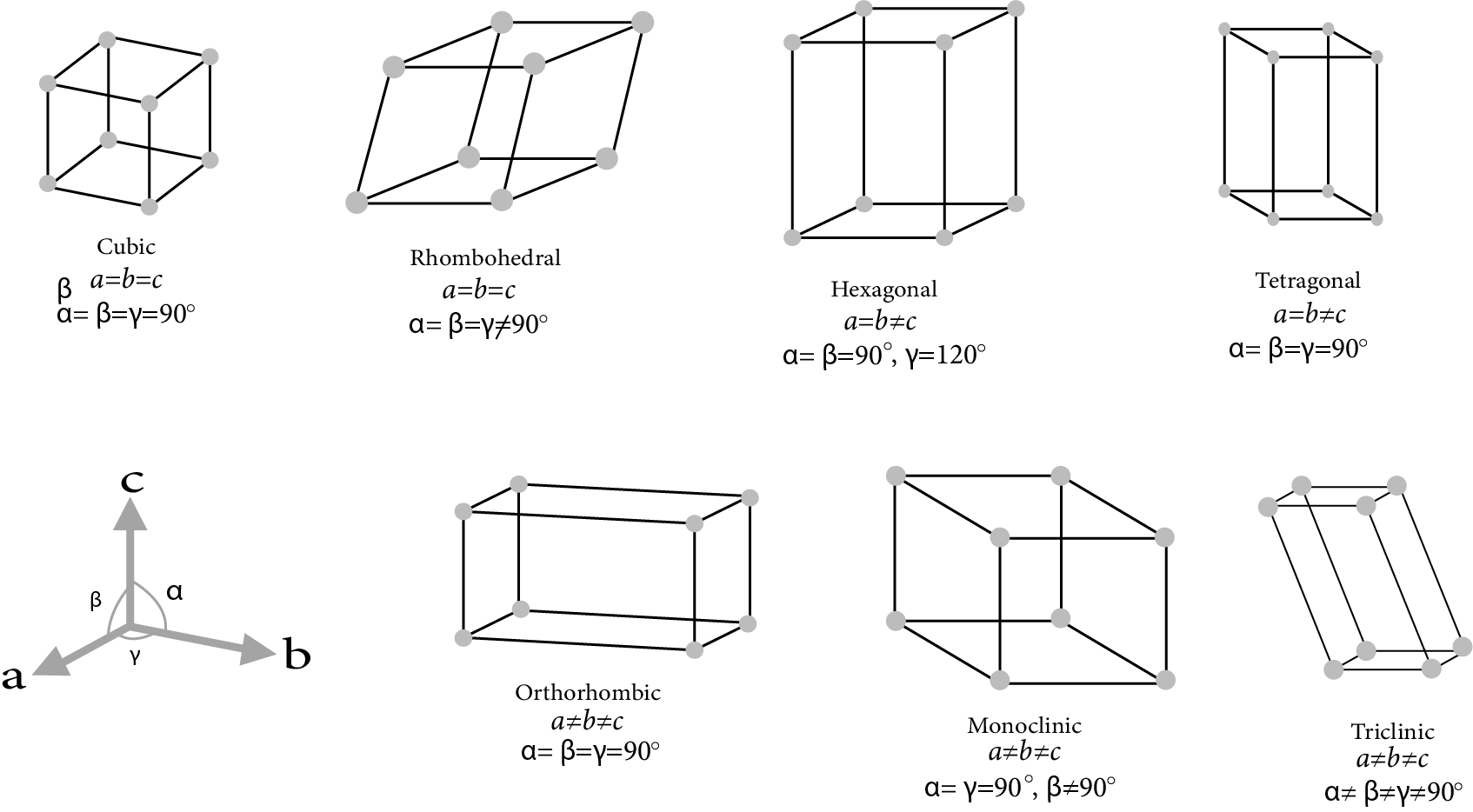
Seven Crystal System
The two tetragonal: one side different length to the other, two angles between face all 90°
The four orthorhombic lattices: unequal sides, angles between faces all 90°
The two monoclinic lattices: unequal sides, two faces have angle different to 90°
Coordination Number for Elemental Crystallization:
The coordination number is the number of nearest such lattice point surrounding a lattice point in a crystalline.
Simple Cubic Cell: In a lattice of this type, each corner atom is shared by eight-unit cells, four in one layer and four in the layer above it.
Thus, the contribution of each atom placed at the corner of the single cubic unit cell is $\dfrac{1}{8} \times 8 = 1$. Thus, the rank of a primitive unit cell is 1.
BCC Unit Cell: In a BCC lattice, each corner atom is shared by 8-unit cells while the body-centred atom is not shared by any other unit cell. So, the number of effective atoms associated with a BCC unit cell is $\left[ {\dfrac{1}{8} \times 8} \right] + 1 = 2$. Thus, the rank of the BCC unit cell is 2.
FCC Unit Cell: In an FCC unit cell, each corner atom is shared by 8-unit cells and each face centred atom is shared by 2-unit cells, so the number of effective atoms in an FCC unit cell would be $\left[ {\dfrac{1}{8} \times 8} \right] + \left[ {\dfrac{1}{2} \times 6} \right] = 4$, thus the rank of FCC unit cell is 4.
HCP Unit Cell: Each corner atom would be common to 6 other unit cells, therefore their contribution to one unit cell would be $\dfrac{1}{6}$, Total number of atom in 1 hcp cell $ = \dfrac{{12}}{6}$(from 12 corners)+$\dfrac{2}{2}$(from 2 face centred)+$\dfrac{3}{1}$(from body centre)=6.
Coordination Number and Radius Ratio
Coordination number and radius ratio are important concepts in solid-state chemistry, particularly in the study of crystal structures and bonding. Here's some content explaining these concepts:
Coordination Number:
The coordination number of an atom in a crystal structure refers to the number of nearest neighbour atoms that are directly bonded to it. It indicates how many atoms are in direct contact with a central atom.
In simple terms, the coordination number is like the number of close friends surrounding a person in a crowd. The more friends surrounding them, the higher the coordination number.
For example, in a cubic crystal lattice like NaCl (sodium chloride), each sodium ion is surrounded by 6 chloride ions, giving it a coordination number of 6. Similarly, each chloride ion is surrounded by 6 sodium ions.
Coordination numbers can vary depending on the crystal structure and the type of bonding present. Common coordination numbers include 4, 6, 8, and 12.
Radius Ratio:
The radius ratio is a ratio used to describe the relative sizes of ions or atoms in a crystal structure. It helps predict the coordination number of an ion in a crystal lattice.
The radius ratio is calculated by dividing the radius of the cation by the radius of the anion (or vice versa), where the cation is the positively charged ion and the anion is the negatively charged ion.
A radius ratio less than 0.155 generally predicts a coordination number of 2.
A radius ratio between 0.155 and 0.225 generally predicts a coordination number of 4.
A radius ratio between 0.225 and 0.414 generally predicts a coordination number of 6.
A radius ratio between 0.414 and 0.732 generally predicts a coordination number of 8.
A radius ratio greater than 0.732 generally predicts a coordination number of 12.
Importance of Coordination Number and Radius Ratio:
These concepts help in understanding and predicting the crystal structures of various compounds.
By knowing the coordination number and radius ratio, we can predict the geometry of crystal lattices and understand the arrangement of atoms or ions in solids.
They are crucial for understanding properties like mechanical strength, electrical conductivity, and optical behaviour of materials.
Example:
Consider the crystal structure of cesium chloride (CsCl). Cesium has a coordination number of 8, and chloride has a coordination number of 8 as well. The radius ratio of CsCl is 0.414, which corresponds to a coordination number of 8 according to the radius ratio rule.
Coordination Numbers | Geometry | Radius Ratio (x) | Example |
2 | Linear | x<0.155 | BeF2 |
3 | Planar Triangle | 0.155 ≤ x <0.225 | AlCl3 |
4 | Tetrahedron | 0.225 ≤ x <0.414 | ZnS |
4 | Square Planar | 0.414 ≤ x <0.732 | PtCl42- |
6 | Octahedron | 0.414 ≤ x <0.732 | NaCl |
8 | Body Centred Cubic | 0.732 ≤ x <0.999 | CsCl |
Hexagonal Closest Packing of Spheres:
Normal
Diagrammatic view
Interstitial Voids: The empty spaces between the three-dimensional layers are known as holes or voids. The holes are also referred to as interstices. There are three types of holes possible.
Tetrahedral Holes: A hole formed by three spheres in contact with each other of a layer. The holes are also referred to as interstices.
Radius ratio of the tetrahedral void,$\dfrac{{{r_{void}}}}{{{r_{sphere}}}} = 0.225$
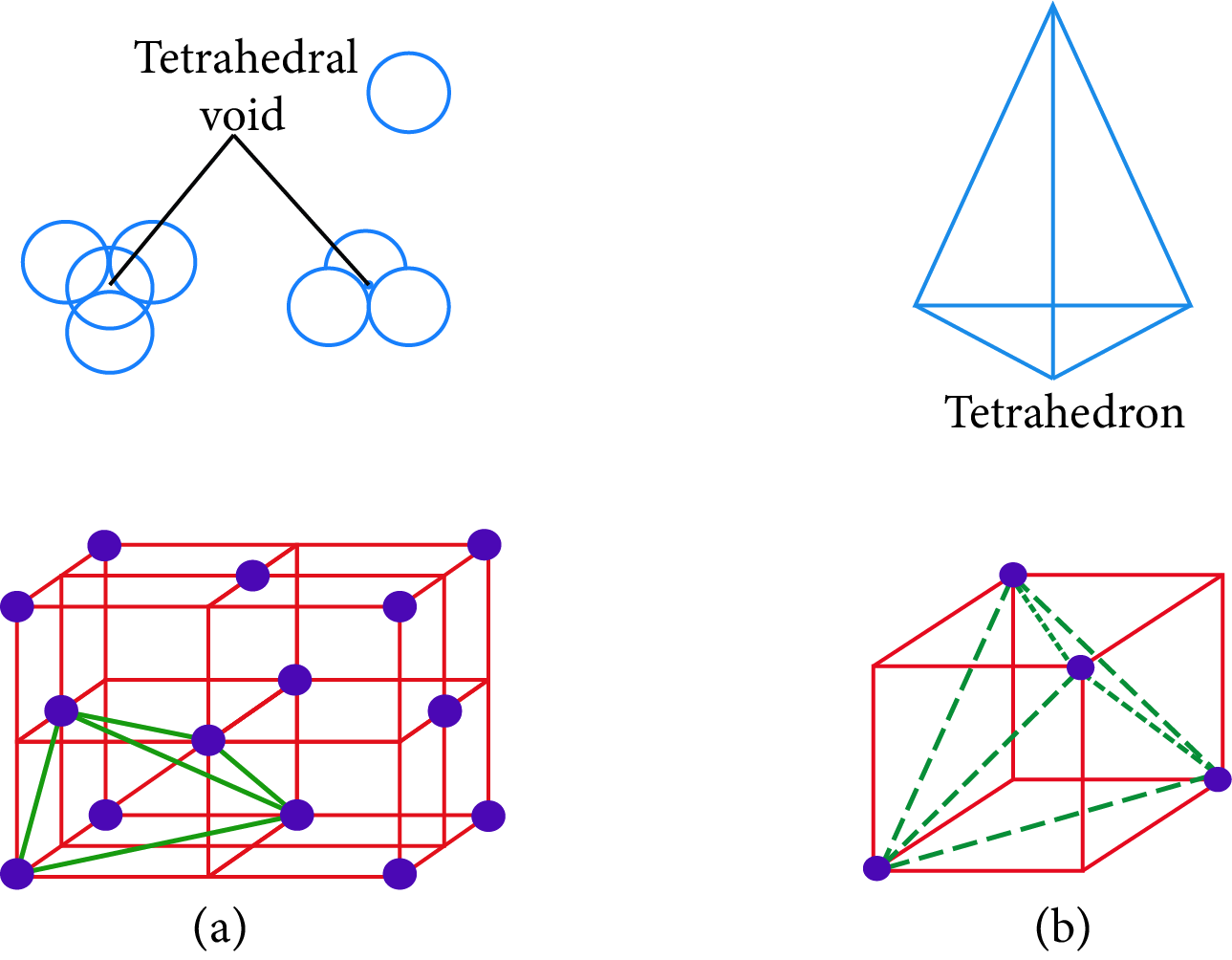
Tetrahedral Hole
Octahedral Hole: It is the vacant space between a group of three-sphere in a layer and another set of three-sphere in the next layer, these six spheres surrounding the hole, lie at the vertices of a regular octahedral.
The total number of octahedral voids $= 1 + 12 \times \dfrac{1}{4} = 1 + 3 = 4$
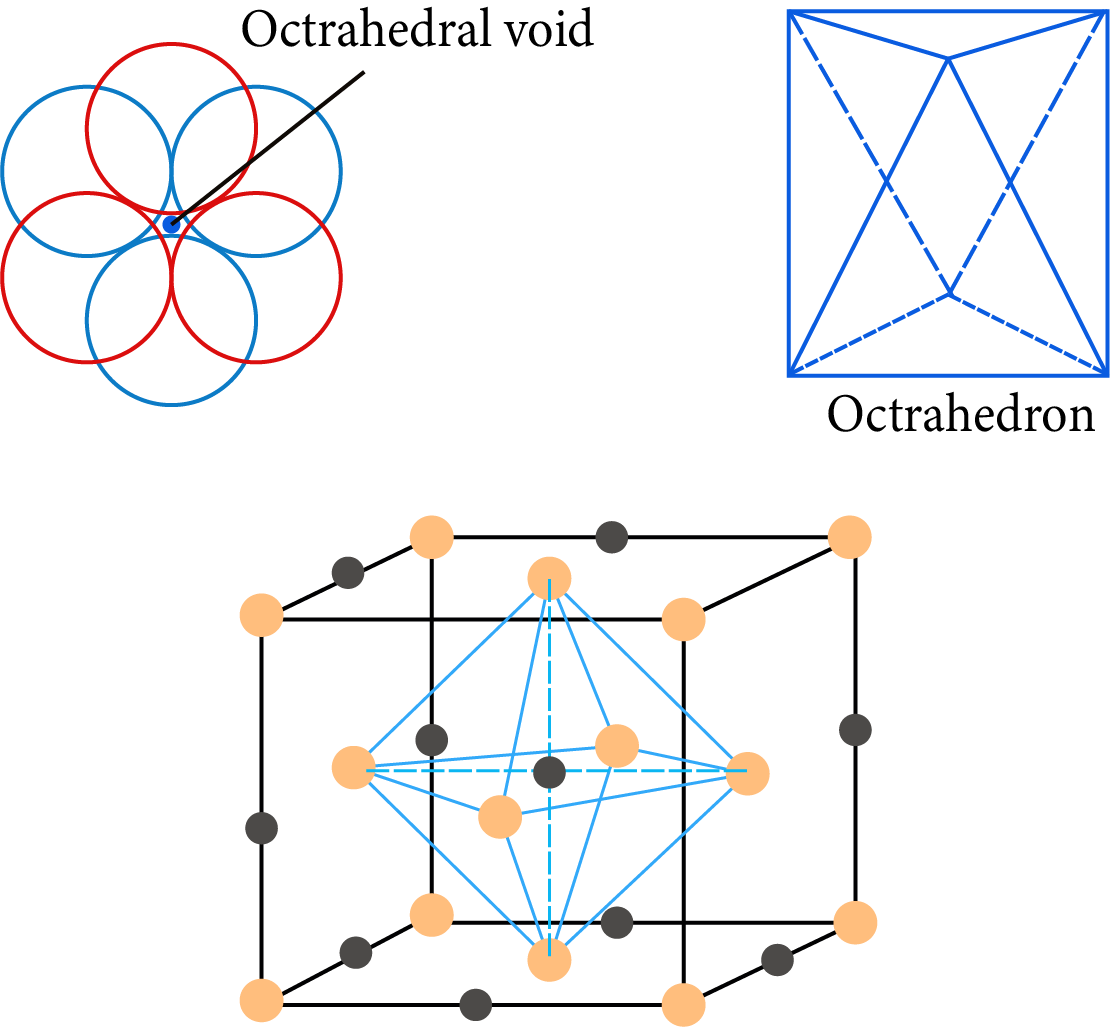
Octahedral Hole
Types of Ionic Structures
The term "ionic structure" refers to the arrangement of positively (Cations) and negatively (Anions) charged ions in a compound. Ionic compounds are formed when atoms of different elements transfer electrons to each other, resulting in the formation of ions. These ions are held together by strong electrostatic forces, forming a three-dimensional lattice structure.
1. Rock Salt Structure (NaCl):
In the rock salt structure, chloride ions (Cl⁻) occupy both the corners and the face centres of a face-centered cubic (FCC) lattice, while sodium ions (Na⁺) occupy the octahedral voids within the lattice.
Both sodium and chloride ions have a coordination number of 6.
2. Zinc Blende (Sphalerite) Structure (ZnS):
In the zinc blende structure, sulfide ions (S²⁻) occupy the main lattice sites, while zinc ions (Zn²⁺) are located in the alternate tetrahedral voids of the FCC crystal lattice.
The coordination number of sulfide and zinc ions is 4.
3. Fluorite Structure (CaF₂):
In the fluorite structure, calcium ions (Ca²⁺) occupy the main lattice positions, and fluoride ions (F⁻) occupy the tetrahedral voids within the FCC crystal structure.
The coordination number of calcium ions is 8, while that of fluoride ions is 4.
4. Anti-Fluorite Structure:
Commonly found in alkali oxides like Na₂O and Li₂O, the anti-fluorite structure consists of oxide ions (O²⁻) occupying the FCC lattice positions, while lithium ions (Li⁺) are located in the tetrahedral voids.
The coordination number of lithium ions is 4, and that of oxide ions is 8.
5. Cesium Halide Structure (CsCl):
In the cesium halide structure, chloride ions (Cl⁻) are positioned at the corners of the unit cell, while cesium ions (Cs⁺) are located at the center of the cell.
Both chloride and cesium ions have a coordination number of 8.
6. Corundum Structure (Al₂O₃):
In the corundum structure, oxygen ions (O²⁻) form a hexagonal close-packed (hcp) lattice, while aluminum ions (Al³⁺) occupy 2/3 of the octahedral voids within the lattice.
7. Rutile Structure (TiO₂):
In the rutile structure, oxygen ions (O²⁻) form a hcp lattice, and titanium ions (Ti⁴⁺) occupy half of the octahedral voids within the lattice.
8. Perovskite Structure (CaTiO₃):
In the perovskite structure, calcium ions (Ca²⁺) are located at the corners of the unit cell, oxygen ions (O²⁻) are positioned at the face centers, and titanium ions (Ti⁴⁺) are situated at the centre of the unit cell.
Packing Fraction in Element Crystallization:
Percentage efficient of simple cubic unit cell =$\dfrac{{{\text{volume of one sphere}}}}{{{\text{volume of unit cell}}}}{\text{ }\times 100}$
${a^3}$= volume of the unit cell
$a = 2{r_s}$ where ${r_s}$ is the radius of the sphere.
$\dfrac{{\dfrac{4}{3}\pi \times {{({r_s})}^3}}}{{{a^3}}} \times 100 = \dfrac{{\dfrac{4}{3}\pi \times {{({r_s})}^3}}}{{{{\left( {2 \times {r_3}} \right)}^3}}} \times 100$
$= \dfrac{{\dfrac{4}{3}\pi }}{8} \times 100 = 52.3$
Percentage efficiency of body-centred unit cell =$\dfrac{{{\text{volume of the two sphere}}}}{{{\text{Volume of cube}}}}{\text{ }\times 100}$
${a^3}$= volume of the unit cell by taking edge length ‘a’
$a = 2{r_s}$where ${r_s}$ is the radius of the sphere.
\[\dfrac{{2 \times \dfrac{4}{3}\pi {{({r_s})}^3}}}{{{a^3}}} \times 100 = \dfrac{{2 \times \dfrac{4}{3}\pi {{({r_s})}^3}}}{{{{\left( {\dfrac{4}{{\sqrt 3 }}{r_s}} \right)}^3}}} \times 100 = 68.05\]
The volume occupied by spheres in a body-centred unit cell is 68.05% and void volume is 31.95%
Length of facial diagonal -$\sqrt{2a}$
Length of body diagonal -$\sqrt{3}a$
Efficiency of packing in face-centred unit cell = $\dfrac{{{\text{volume of the four sphere}}}}{{{\text{Volume of cube}}}}{\text{ }\times 100}$
${a^3}$= volume of the unit cell by taking edge length ‘a’
$a = 2{r_s}$where ${r_s}$ is the radius of the sphere.
\[\dfrac{{4 \times \dfrac{4}{3}\pi {{({r_s})}^3}}}{{{a^3}}} \times 100 = \dfrac{{4 \times \dfrac{4}{3}\pi {{({r_s})}^3}}}{{{{\left( {2\sqrt 2 {r_s}} \right)}^3}}} \times 100 = 74.06\]
Thus, the volume occupied by a sphere in face-centred unit cell (cpp arrangement) is 74.06% and the void volume is 25.94%
Volume Fraction in HCP:
$V.F = \dfrac{{6 \times \dfrac{4}{3}\pi {R^3}}}{{{V_{HCP}}}}$
${{\text{V}}_{{\text{HCP}}}}{\text{ = Area of base }\times Height}$
${\text{ = 3}}\sqrt {2{a^3}} = 24\sqrt 2 {R^3}$
Volume fraction of HCP=$4\sqrt {\dfrac{2}{3}} R$
Note: Distance between A and B layer in CCP or HCP=$2\sqrt {\dfrac{2}{3}} R$
Bragg’s Equation: $n\lambda = 2d\sin \theta $
n= order of reflection
$\lambda$=wave length of incident x-ray
d= interplanar distance
$\theta$=angle of reflection
Types of Crystal Defects:
Intrinsic Defect: These are seen in pure crystal.
Extrinsic Defects: These are due to the impurities in the solids.
Point Defect: These Occur at the lattice points or sites in the crystals.
Extended Crystal: These are present in one or more dimensions.
Stoichiometric Defects:
Ionic Solids Show Two Types of Defects
Schottky Defect
This defect arises due to vacancy at cation sites and equal number of vacancies at anion sites
Condition for the defect:
High co-ordination numbers and where the positive and negative ions are of similar size, i.e., $\dfrac{{{r^ + }}}{{{r^ - }}} \cong 1$
Number of Schottky defect
$n = N{e^{ - E/2kt}}$
$n = {\left( {N{N_i}} \right)^{1/2}}{e^{ - E/2kt}}$
K = Boltzmann’s constant
T= Kelvin temperature
E= energy required to create defect
N= number of ions present
Ni= number of interstitial spaces
Frenkel Defect
This type of defect arises due to a vacancy at a cation site.
Conditions that favour the defect:
Large difference in size between cation and anion and Compounds having ions of different size i.e., $\dfrac{{{r^ + }}}{{{r^ - }}}$ is low since positive ions are smaller than negative ions.
Doping:
Addition of B(IIIA Group) or P or As (VA Group) element to alter the conductivity of Ge(or) Si is called Doping.
Pure Si (or) Ge are an intrinsic semiconductor.
In doping, group IIIA(13 group element) elements behave as electrons acceptor and group VA(15 group elements) elements behave as donor.
Key Points to Remember
They possess high density, rigidity, low compressibility, and a definite shape and volume.
Solid classifications include ionic, molecular, covalent, and metallic, depending on the bonding involved.
Solids are characterized by their rigid nature, maintaining a fixed shape and volume.
Solids are categorized based on their properties and the type of bonding present in their constituent particles.
Elevated temperatures can lead to the creation of defects in solids, such as stoichiometric defects, non-stoichiometric defects, or impurity defects. These defects alter the properties of the solid.
Crystalline solids exhibit an ordered arrangement of atoms, molecules, or ions over a long range, while amorphous solids have randomly arranged particles without a long-range order.
The crystal lattice represents the symmetrical three-dimensional arrangement of atoms, ions, or molecules within crystalline solids, forming points.
Packing fraction, or packing efficiency, calculates the ratio of the volume occupied by spheres within a unit cell to the total volume of the unit cell.
The unit cell is the fundamental repeating unit within the crystal lattice.
The radius ratio indicates the ratio between the smaller ionic radius (cation) and the larger ionic radius (anion) within a compound.
The coordination number signifies the number of nearest neighbour atoms, ions, or molecules surrounding a central atom or ion within a crystal or complex.
Voids refer to the empty spaces between constituent particles in closed-packed structures.
Understanding these key points is crucial for comprehending the properties and behaviour of various solid materials.
Importance of Physical Chemistry Solid State
Here you will learn the different aspects of the Solid State at an advanced level. This chapter will explain how the molecules form solid and what their characteristics are.
Go through these notes to quickly revise the physical properties of a solid and realize how this state of matter is defined. With the knowledge of the physical properties of different solids, differentiate, distinguish, and identify different materials. This chapter will also describe the classification of solids following the various features.
The importance of this chapter is that it explains the different physical properties of all classes of solids with examples. All these classifications are done based on particular parameters such as crystal structure and chemical bonding. It will also explain how lattices are formed and what kinds of lattices exist with pictorial illustrations. Get Solid State for JEE Advanced key concepts from the chapter and refer to these notes for a quick revision.
Benefits of Vedantu’s Solid State JEE Notes PDF
The revision notes offer a simpler explanation of all the advanced concepts of this chapter in an easy-to-understand language. You will find it easier to learn different types of solids, types of crystalline structures, their examples, and all other topics included in this chapter.
The simpler version of this chapter in the Solid State JEE notes PDF will help you complete preparing this chapter in no time. You will find learning the fundamental principles much easier. In fact, you can also resolve doubts on your own and proceed to solve exercise questions without any difficulty.
These revision notes are the easiest way to revise the chapter before the exam date. These notes also come with Solid State JEE Advanced questions and answers prepared by the experts. Students can try to solve the questions on their own and check their preparation level.
Students can easily recall all the concepts, definitions, derivations, formulas, etc of this chapter by using these notes to score well in the competitive exam.
Examples with Solutions
1. Lithium metal crystallises in a body-centred cubic crystal. If the length of the side of the unit cell of lithium is 351 pm, the atomic radius of the lithium will be:
A. 151.8 pm
B. 75.5 pm
C. 300.5 pm
D. 240.8 pm
Answer: Given that lithium metal crystallises in a body-centered cubic crystal, the atomic radius,
r = (√3/4) x a
Here, a is the edge length
r = (√3/4) x 351
= 151.8 pm
2. Silver (atomic weight = 108 g mol-1) has a density of 10.5 g cm-3. The number of silver atoms on a surface of an area of 10-12 m2 can be expressed in scientific notation as y × 10x. The value of x is:
A. 3
B. 5
C. 7
D. 9
Answer: Density of the crystal = 10.5 g cm-3
Silver forms a face-centered cubic (FCC) unit cell containing four atoms per unit cell when it crystallizes.
ρ = (4 x 108)/ (6.023 x 1023 x a3)
10.5 = (4 x 108)/ (6.023 x 1023 x a3)
⇒ a3 = 6.83 x 10-23 cm3
a = 4 x 10-8 cm
= 4 x 10-10 m
The surface area of the unit cell
⇒ a2 = 1.6 x 10-19 m2
Number of unit cells on 10-12 m2 surface
= 10-12/ (1.6 x 10-19)
= 6.25 x 106
One face of the unit cell contains two atoms.
Number of atoms on 10-12 m2 surface = 2 x number of unit cell = 1.25 x 107
⇒ x = 7
3. A solid compound contains X, Y and Z atoms in a cubic lattice with X atoms occupying the corners. Y atoms in the body-centred positions and Z atoms at the centres of faces of the unit cell. What is the empirical formula of the compound?
A. XY2Z3
B. XYZ3
C. X2Y2Z3
D. X8YZ6
E. XYZ
Answer: To find the empirical formula of the compound, we need to determine the ratio of X, Y, and Z atoms in the unit cell.
Atoms at Corners (X):
Each corner atom contributes ⅛ to the unit cell.
There are 8 corners in a cube, so the contribution of X atoms is 8×⅛ =1
Atoms in Body-Centered Positions (Y):
Each body-centered atom contributes 1 to the unit cell.
There is 1 body-centered atom in a cube.
Atoms at the Centers of Faces (Z):
Each face contains 1/2 of a Z atom.
There are 6 faces in a cube, so the total contribution of Z atoms is 6×½ =3
Now, let's calculate the ratio of X, Y, and Z atoms in the unit cell:
X : Y : Z = 1 : 1 : 3
Therefore, the empirical formula of the compound is X1Y1Z3, which is simplified to XYZ3
4. Structure of a mixed oxide is cubic close-packed (c.c.p). The cubic unit cell of mixed oxide is composed of oxide ions. One-fourth of the tetrahedral voids are occupied by divalent metal A, and the octahedral voids are occupied by a monovalent metal B. The formula of the oxide is:
A. ABO2
B. A2BO2
C. A2B3O4
D. AB2O2
Answer: To determine the formula of the oxide, let's analyse the occupation of voids by metals A and B in the cubic close-packed (ccp) structure:
Tetrahedral Voids (Occupied by Metal A):
Each ccp unit cell has 8 tetrahedral voids.
One-fourth of the tetrahedral voids are occupied by metal A.
Therefore, the number of metal A ions in the unit cell = 8 × (1/4) = 2.
Octahedral Voids (Occupied by Metal B):
Each ccp unit cell has 4 octahedral voids.
All octahedral voids are occupied by metal B.
Therefore, the number of metal B ions in the unit cell = 4.
Now, let's use these numbers to determine the empirical formula:
Metal A (divalent) contributes 2 cations.
Metal B (monovalent) contributes 4 cations.
Oxide ions contribute 4 anions (ccp structure).
So, the empirical formula should have the ratio of cations to anions: A2B4O4
However, we need to simplify this formula to the most reduced form. Dividing all subscripts by 2 gives: AB2O2
5. Total volume of atoms present in a face-centred cubic unit cell of metal is (r = atomic radius)
A. 20/3 πr3
B. 24/3 πr3
C. 12/3 πr3
D. 16/3 πr3
Answer: The face-centered cubic (fcc) structure contains four atoms within each unit cell.
The volume of an atom (V) = (4/3)πr3
In fcc, The total volume of atoms = 4 x V
= 4 x (4/3)πr3
= (16/3)πr3
6. The three states of matter are solid, liquid and gas. Which of the following statements is true about them?
A. Gases and liquids have viscosity as a common property
B. The molecules in all three states possess random translational motion
C. Gases cannot be converted into solids without passing through the liquid phase
D. Solids and liquids have pressure as a common property
Answer: Viscosity is a property shared by both gases and liquids.
Problems for Practice
1. The pure crystalline substance, on being heated gradually, first forms a turbid liquid at constant temperature and still, at a higher temperature, turbidity completely disappears. The behaviour is a characteristic of substance forming.
A. Allotropic crystals
B. Liquid crystals
C. Isometric crystals
D. Isomorphous crystals
2. A closed flask contains water in all its three states solid, liquid and vapour at OoC. In this situation, the average kinetic energy of water molecules will be:
A. The greatest in all the three states
B. The greatest in the vapour state
C. The greatest in the liquid state
D. The greatest in the solid-state
3. A solid has a structure in which W atoms are located at the corners of a cubic lattice, O atoms at the centre of the edges and Na atoms at the centre of the cube. The formula for the compound is:
A. NaWO2
B. NaWO3
C. Na2WO3
D. NaWO4
4. In the laboratory, sodium chloride is made by burning sodium in the atmosphere of chlorine. The salt obtained is yellow in colour. The cause of the yellow colour is:
A. Presence of Na+ ions in the crystal lattice
B. Presence of Cl– ions in the crystal lattice
C. Presence of electrons in the crystal lattice
D. Presence of face-centred cubic crystal lattice
5. In a face-centred cubic arrangement of A and B atoms in which A atoms are at the corners of the unit cell, and B atoms at the face centres, one of the A atoms is missing from one corner of the unit cell. The simplest formula of the compound is:
A. A7B3
B. AB3
C. A7B24
D. A7/8B3
6. X-rays of wavelength equal to 0.134 nm give the first-order diffraction from the surface of a crystal when the value of θ is 10.5o. The distance between the planes in the crystal parallel to the surface is (sin 10.5o = 0.1822):
A. 0.368 nm
B. 0.184 nm
C. 0.726 nm
D. 0.92 nm
Download Solid State JEE Revision Notes PDF
Students can add the free PDF version of these revision notes and questions to their JEE Advanced preparation material for this chapter. Many students prefer to use these notes to make their study sessions more productive. The simpler format of these notes will help them to recall the definitions, explanations, formulas, and derivations of this chapter without any difficulty.
Important Related Links for JEE Main and JEE Advanced
FAQs on JEE Advanced 2026 Solid State Notes
1. What is perfection in solids?
The crystal structure of a solid remains in perfect order at 0 K. It is called perfection in the solid state. The electrons start to move randomly as the temperature gradually increases. It results in an imperfect stage.
2. What are insulators?
As per the definition in this chapter, a solid that does not allow electrons or heat energy to pass through is called an insulator. Example: plastic, Bakelite, etc.
3. What are diamagnetic substances?
These are magnetic substances that are weakly attracted by magnets. They contain paired electrons in the outermost shells. Example: titanium dioxide and sodium chloride.
4. What is an amorphous solid?
The solids that do not have an even crystalline shape with no sharp edges are called amorphous solids. These solids do not have a proper melting point and heat of fusion.
5. What are the important points of solids?
Important points of solids include:
Definite shape and volume
Particles are closely packed in a regular pattern
Strong intermolecular forces
High density compared to gases and liquids
Low compressibility
6. What are the important points of solid state NCERT?
Important points from the NCERT (National Council of Educational Research and Training) regarding solid state include:
Classification of solids into crystalline and amorphous
Crystal lattice and unit cell
Types of crystal lattices (cubic, tetragonal, etc.)
Imperfections in solids (vacancies, interstitials, etc.)
Electrical, magnetic, and optical properties of solids
7. What are the properties of solids and liquids notes?
Properties of solids and liquids include:
Solids have definite shape and volume, while liquids have definite volume but take the shape of their container
Solids have strong intermolecular forces, while liquids have weaker forces
Solids are less compressible compared to liquids
Solids generally have higher densities than liquids
Solids usually have higher melting points than liquids
8. What are the prerequisites for solid state chemistry?
Prerequisites for solid state chemistry include:
Understanding of atomic structure and chemical bonding
Knowledge of periodic table and periodic trends
Basic understanding of crystallography and symmetry
Familiarity with concepts of thermodynamics and kinetics
Proficiency in stoichiometry and chemical equations
9. What are the special applications of solid-state materials?
Special applications of solid-state materials include:
Semiconductor devices like transistors and diodes
Superconductors for magnetic levitation and efficient power transmission
Piezoelectric materials for sensors and actuators
Magnetic materials for data storage and medical imaging
Optoelectronic materials for LEDs, lasers, and solar cells
10. What is a good example of solid-state storage?
A good example of solid-state storage is the Solid State Drive (SSD). SSDs use semiconductor memory to store data persistently without the need for mechanical parts like those found in traditional hard disk drives (HDDs). They offer faster data access, improved reliability, and lower power consumption compared to HDDs.
























