




How Does Millon’s Reagent Detect Proteins?
Millon's test was discovered by the French Chemist Auguste Nicolas Eugene Millon. Millon’s test is predicated on the principle of nitrification of the phenol group in tyrosine, which then forms complexes with significant metals like mercury. A reagent may be a compound or mixture added to a system to begin or check a chemical change.
A reagent may be used to confirm the presence or absence of a selected chemical substance as the binding of reagents to the substance or different connected substances triggers bound reactions. The reagent used for the test is Millon’s reagent, consisting of metal nitrate of mercury and mercuric nitrate that is dissolved in concentrated nitric acid.
What is a Reagent?
In terms of chemistry, a reagent is an organic or inorganic substance that triggers a series of chemical reactions once added to a combination. Reagents are used to test the presence of different functional groups in a solution. This makes various types of reagents helpful for testing functions.
The term reagent means a chemical ingredient that, once added to an organic mixture, transforms that mixture into another variety of substances. A few examples of reagents used are:
1. Collin’s Reagent
This reagent may contain Chromium(IV) pyridine in dichloromethane. It's a solid red colour compound and extremely helpful in oxidisation.
2. Grignard Reagent
This cluster has the formula \[\text{RMgX}\] , wherever X is halogen and R is alkyl or aryl group.
3. Millon’s Reagent
A few drops of this chemical agent will detect the presence of soluble proteins in a test solution. Once this happens, the chemical agent turns reddish brown or precipitates are shaped to confirm the tyrosine residue's presence.
Millon’s Reagent
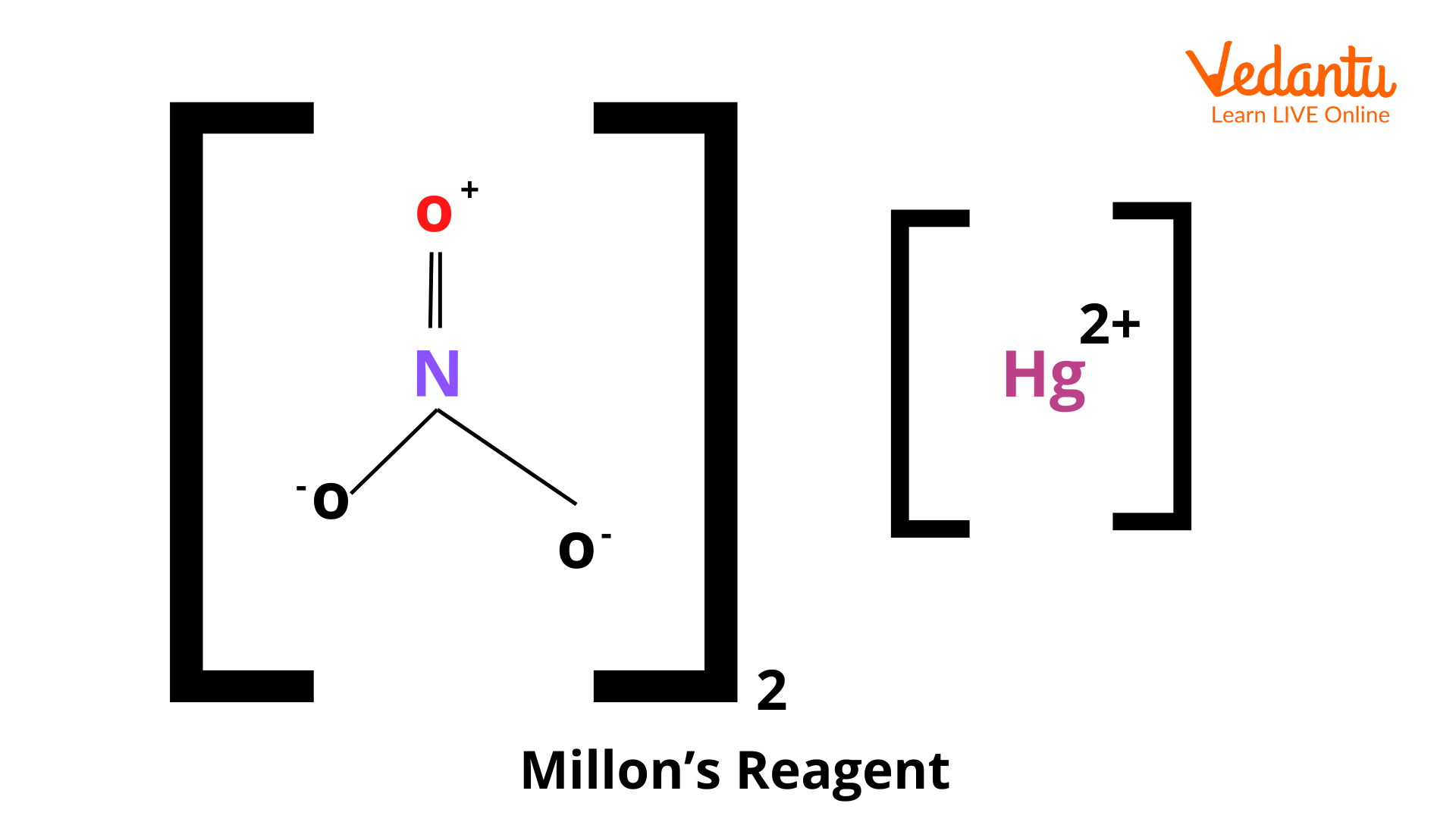
Millon's reagent is an analytical reagent used to notice the presence of soluble proteins. Some drops of the chemical agent are added to the test mixture and then heated gently. A reddish-brown colouration or precipitate indicates the presence of tyrosine residue in nearly all proteins.
\[\left( \text{Hg}{{\left( \text{N}{{\text{O}}_{\text{3}}} \right)}_{\text{2}}} \right)\] is the formula of Millon’s reagent. The preparation of Millon’s reagent is done by dissolving metallic mercury in acid and diluting it with water, forming mercurous nitrate \[\left( \text{Hg}{{\left( \text{N}{{\text{O}}_{\text{3}}} \right)}_{\text{2}}} \right)\]. within the test, the phenol functional group in the side chain of tyrosine gets nitrated, and the product then complexes with \[\text{Hg}\left( \text{I} \right)\] or \[\text{Hg}\left( \text{II} \right)\] ions to convey a red-coloured precipitate.
Millon’s Reagent
Composition of Millon’s Reagent
The Millon’s reagent composition is as follows:
Mercuric Nitrate
Mercurous Nitrate
Concentrated Nitric Acid
Distilled water
Principle of Millon’s Test
Millon’s test depends on the principle of nitrification of the phenol cluster in amino acid, which then forms complexes with significant metals like mercury. The agent used for the test is termed Millon’s agent.
In the test, the phenol functional cluster on the amino acid molecule is nitrated by the acid present within the agent. The nitrated amino acid then combines with the mercury ions within the mixture to make a red-coloured precipitate or solution.
In some proteins containing amino acids, the initial reaction between metal nitrate ends up in a white or yellow-coloured precipitate. With the addition of acid, followed by heating, the residue turns red. Each of these results is believed to have positive results and indicates the presence of amino acids within the solution.
The Objective of Millon’s Test
1. To observe the presence of tyrosine-containing proteins in a very given sample
2. To observe the presence of phenol-containing compounds
3. To entirely differentiate tyrosine from different amino acids
Materials Needed
Test tubes
Test tube stand
Pipettes
Water bath
The Procedure of Millon’s Test
1. A certain quantity of the sample mixture or the amino acid solution is taken within the tube.
2. Millon’s agent is added to the current mixture. The test tubes are then unbroken within the water bathtub for several minutes if the red-coloured precipitate isn't discovered directly.
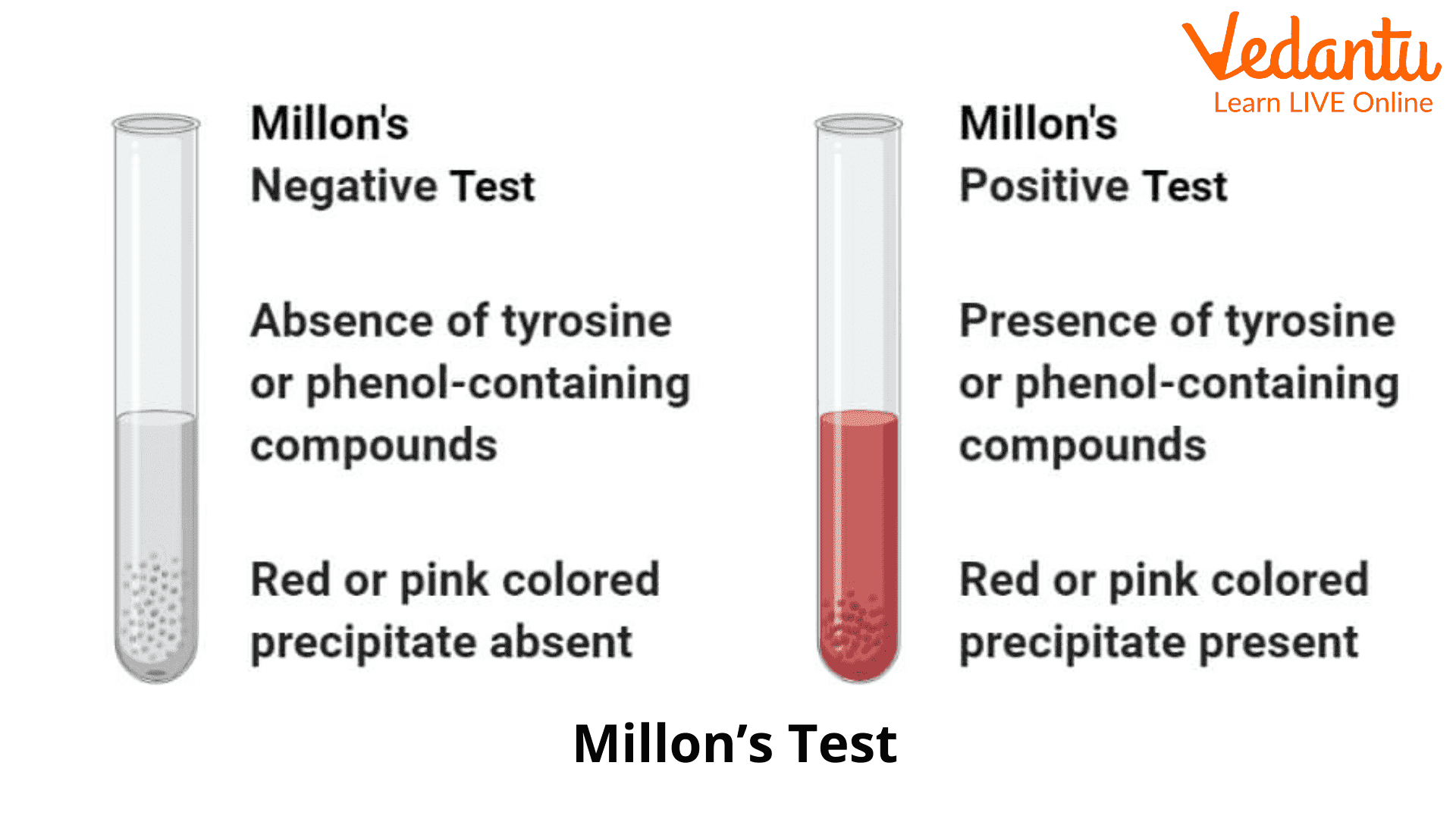
3. The tubes are then observed for the formation of the coloured precipitate.
Millon’s Test
Millon’s test reactions are discussed as follows:
Positive Result: Millon’s test demonstrates a positive end by forming a red or pink-coloured precipitate. This suggests the presence of amino acids or amino acids containing supermolecules.
Negative Result: Millon’s test demonstrates a negative end by the absence of coloured precipitate in the tube. This suggests the absence of amino acids or tyrosine-containing supermolecules.
Uses of Millon's Reagent
1. Millon’s test is employed to detect tyrosine-containing proteins in an exceedingly given sample.
2. The test conjointly helps in the differentiation of amino acids from one another.
3. The test is helpful in the detection of casein macromolecules and also the macromolecules found in meat.
Limitations of Millon’s Test
1. Compounds like 2-hydroxybenzoic acid and phenoplast compounds provide a positive result for the present test; therefore, the other phenol compounds present within the tube should be avoided. Tests like the Biuret and Ninhydrin tests should be performed for confirmation.
2. The presence of gas within the solution may interfere with the reaction; therefore, the test can not be performed on a sample containing chlorides.
3. The formation of a white or yellow precipitate may well be discovered right away once the addition of Millon’s chemical agent because of the denaturation of proteins by mercurous ions.
Summary
Millon’s reagent and its preparation deal with the chemical composition of the reagent. A reagent is an organic or inorganic substance that triggers a series of chemical reactions once added to a combination. Reagents are used to test the presence of different functional groups in a solution. Millon's reagent is an analytical reagent used to notice the presence of soluble proteins. The limitations and uses of Millon’s test have been discussed in this article.
FAQs on Millon’s Reagent: Preparation, Properties & Applications
1. What is Millon's reagent and what is its primary use in chemistry?
Millon's reagent is an analytical solution used in biochemistry to detect the presence of soluble proteins. Its primary purpose is to identify the amino acid Tyrosine, which contains a phenolic group. When a sample containing Tyrosine is heated with Millon's reagent, it produces a distinct brick-red precipitate, indicating a positive test.
2. How is Millon's reagent prepared in a laboratory setting?
The preparation of Millon's reagent involves dissolving metallic mercury in nitric acid. The typical procedure is as follows:
- One part of metallic mercury (Hg) is dissolved by weight in two parts of concentrated nitric acid (HNO₃). This should be done carefully, often in a fume hood, as toxic fumes are released.
- Once the mercury has completely dissolved, the resulting solution is diluted with two volumes of distilled water.
- The solution is allowed to stand, and the clear supernatant liquid is decanted to be used as Millon's reagent.
3. What is the precise chemical composition of Millon's reagent?
Millon's reagent is not a single compound but a solution containing several active components. Its composition is a mixture of mercury(I) nitrate (Hg₂(NO₃)₂) and mercury(II) nitrate (Hg(NO₃)₂) dissolved in a dilute solution of nitric acid (HNO₃). The presence of both mercury ions and nitric acid is essential for the detection reaction.
4. What is the underlying chemical principle of Millon's test?
The principle behind Millon's test is a two-step chemical reaction. First, the phenolic group (a hydroxyl group attached to a benzene ring) in a compound like tyrosine is nitrated by the nitric acid present in the reagent. This forms a nitrated derivative. Subsequently, the mercury ions (Hg⁺ and Hg²⁺) in the reagent coordinate with this nitrated phenol to form a dense, coloured mercury complex, which appears as a red precipitate.
5. Why is Millon's test specific for the amino acid Tyrosine when testing proteins?
Millon's test is specific for Tyrosine among the standard protein-building amino acids because it exclusively detects the presence of a phenolic hydroxy group. Out of the 20 common amino acids that make up proteins, only Tyrosine has this specific chemical structure. Therefore, if a protein gives a positive Millon's test, it confirms that the protein's structure contains Tyrosine residues.
6. What observation confirms a positive result in Millon's test?
A positive result in Millon's test is confirmed by a distinct colour change upon heating. Initially, adding the reagent may form a white precipitate. When this mixture is gently heated, the precipitate will turn from white to a brick-red or pink colour. The formation of this red precipitate is the definitive sign of a positive test.
7. Are there any substances or conditions that can interfere with or cause a false positive in Millon's test?
Yes, there are important limitations. Any non-protein compound that contains a phenolic group, such as phenol or salicylic acid, will also produce a positive red colour, leading to a false positive for proteins. Additionally, the test is inhibited by the presence of chloride ions (Cl⁻), as they will precipitate the mercury from the reagent as insoluble mercury chloride, preventing it from reacting with tyrosine.























