




What is Ammonium Oxalate? Definition, Characteristics & Applications
In Chemistry, salt is known as a substance made by the reaction between acid and base. Ammonium oxalate is an oxalate salt with ammonium (sometimes as a monohydrate). It is a colourless (white) salt under standard conditions and is odourless and non-volatile. It is the ammonium salt of oxalic acid and occurs in many plants and vegetables. Ammonium oxalate is used as an analytical reagent and general reducing agent. It and other oxalates are used as anticoagulants to preserve blood outside the body. Acid ammonium oxalate (ammonium oxalate acidified to pH 3 with oxalic acid) is commonly employed in soil chemical analysis to extract iron and aluminium from poorly-crystalline minerals (such as ferrihydrite), iron (II)-bearing minerals (such as magnetite), and organic matter.
Ammonium Oxalate
A 2:1 ratio of ammonium and oxalate ions is used to produce the ammonium salt known as ammonium oxalate. It is both an oxalate and an ammonium salt. Other names for it include Ethanedioic acid, diammonium salt, Oxalic acid, and diammonium salt. It can be found in a wide variety of plants and vegetables as well.
Ascorbic acid or glyoxylic acid metabolism in the human body also produces it. It is eliminated in the urine rather than being digested. It serves as a general reducing agent and analytical reagent. Ammonium oxalate is an odourless and colourless crystalline powder.
The compound ammonium oxalate can be found in many distinct kidney stone types. It can also be found in seals, seabirds, and/or bat droppings. Since the higher concentration of ammonium oxalate and urate can be utilised as fungicides and fertilisers for plants, the mixture is rich in nitrogen.
Ammonium Oxalate Formula
The chemical formula for ammonium oxalate is NH4OOCCOONH4. The molecular formula for Ammonium oxalate is C2H8N2O4. Ammonium oxalate as the name suggests contains 2 molecules of ammonia and one molecule of oxalate.
Ammonium Oxalate Solution
To make ammonium oxalate solution, first oxalic acid is dissolved in the desired amount of water and then mixed with the required amount of ammonium. Ammonium oxalate is a solid substance that slowly dissolves and combines in water. Oxalic acid is converted into oxalate ion (C2O4)22- by the dissociation of two acidic hydrogens, which results in the formation of an anion. The molecules include two ammonium ions (NH4)+ in the form of cations, with one ammonium replacing each hydrogen lost.
Ammonium Oxalate Test
Oxalic acid can be determined by using a method such as permanganate titration and atomic absorption. As ammonium oxalate helps in preventing or inhibiting the coagulation of blood plasma; hence, it is used in blood tests to prevent blood coagulation. The lead or calcium ions present in blood as well as other analytes can be measured by complexing this chemical with specific metals.
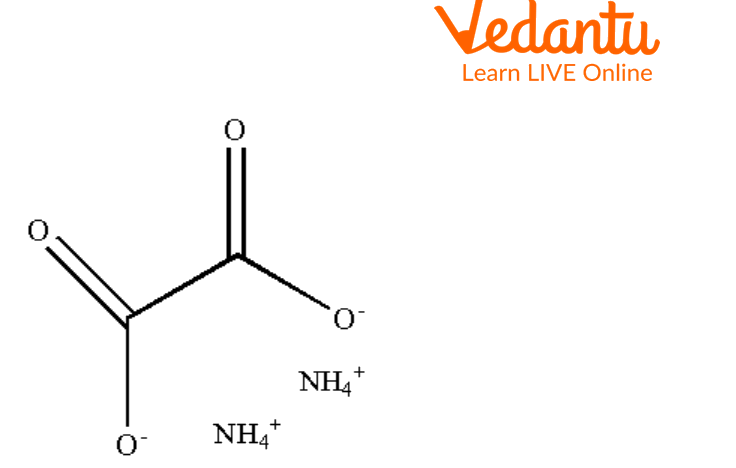
Structure of Ammonium Oxalate
Ammonium Oxalate Uses
Ammonium oxalate can be used in the following ways:
Ammonium oxalate is produced by the metabolism of either glycolic acid or ascorbic acid.
It can be used in mineral form as oxammite.
It can be utilised as an analytical reagent in various tests.
This salt form can also be used as a reducing agent.
It can be used as an anticoagulant for preserving blood in the test tube.
In textile, it is used for dyeing.
There are some side effects of ammonium oxalate as given below:
Ammonium oxalate dust when ingested or inhaled excessively causes systemic poisoning in the body.
Ammonium oxalate when comes in contact with the eyes can irritate. Similarly, contact with skin can irritate severe burns.
Molecular Weight of Ammonium Oxalate
The chemical formula of ammonium oxalate is C2H8N2O4.
So, (12×2) + (1×8) + (14×2) + (16×4) =124.1
Thus, the molecular weight of ammonium oxalate is 124.1 g/mol.
Ammonium Oxalate Monohydrate [(NH4)2C2O4]
The molecular weight of ammonium oxalate monohydrate is 142.11 g/mol. Same as ammonium oxalate, it is also used for the detection of lead and calcium. Ammonium oxalate monohydrate is most famously used as a buffering agent. The monohydrate is the main commercial form of ammonium oxalate.
Interesting Facts
Ammonium oxalate is an odourless solid. Sinks and mixes slowly with water.
Ammonium oxalate is a salt that is made up of ammonium and oxalic acid in the ratio of 2:1. It is colourless, odourless, and soluble in water.
Key Features of Ammonium Oxalate
Ammonium oxalate is used to make explosives and metal polishes and in textile dyeing and analytical chemistry.
It is used as a buffering agent, anticoagulatory for blood and determination of lead calcium, etc.
Calcium chloride solution forms a precipitate of calcium oxalate with ammonium oxalate.
Ammonium oxalate is usually acidic in nature. The acidity depends on the amount of oxalic acid present.
FAQs on Ammonium Oxalate: Properties, Uses, and Structure
1. What is ammonium oxalate and what is its chemical formula?
Ammonium oxalate is an inorganic salt formed from ammonium cations and oxalate anions. It is a colourless, odourless solid at room temperature. Its chemical formula is (NH₄)₂C₂O₄.
2. How is ammonium oxalate typically prepared?
Ammonium oxalate is prepared through a neutralization reaction. This is done by reacting oxalic acid (H₂C₂O₄) with an ammonium source, such as ammonium hydroxide (NH₄OH). The reaction produces ammonium oxalate and water.
3. What are the main uses of ammonium oxalate?
Ammonium oxalate has several important applications, including:
- As an anticoagulant: It is used in blood collection tubes to prevent blood from clotting by binding with calcium ions.
- As an analytical reagent: It is used in labs to detect and measure the concentration of calcium ions.
- In manufacturing: It is used in the production of some explosives, metal polishes, and dyes.
4. Is ammonium oxalate an ionic or covalent compound?
Ammonium oxalate is an ionic compound. It is formed by the electrostatic attraction between two polyatomic ions: the positively charged ammonium cation (NH₄⁺) and the negatively charged oxalate anion (C₂O₄²⁻).
5. What are the safety risks associated with ammonium oxalate?
Ammonium oxalate is toxic and should be handled with care. Inhaling its dust or ingesting it can be harmful. Direct contact may cause severe irritation to the skin and eyes. It is important to use protective gear like gloves and goggles when working with it.
6. How exactly does ammonium oxalate stop blood from clotting?
Blood clotting requires the presence of free calcium ions (Ca²⁺). The oxalate ions from ammonium oxalate bind strongly with these calcium ions to form an insoluble precipitate called calcium oxalate. By effectively removing the calcium ions from the blood plasma, it interrupts the clotting cascade and prevents the blood sample from coagulating.
7. What is the difference between ammonium oxalate and ammonium oxalate monohydrate?
The main difference is the presence of water in the crystal structure. Ammonium oxalate monohydrate, with the formula (NH₄)₂C₂O₄·H₂O, is the hydrated form, meaning each formula unit is chemically bound to one molecule of water. The term 'ammonium oxalate' often refers to this common hydrated form, while the anhydrous form has no water molecules.
8. Why is ammonium oxalate solution a key reagent for detecting calcium in a lab test?
Ammonium oxalate is used to test for calcium because it provides a very clear and specific result. When an ammonium oxalate solution is added to a sample containing calcium ions, it forms a distinct white, crystalline precipitate of calcium oxalate. This visible reaction is a reliable confirmation of the presence of calcium, making it a standard tool in qualitative analysis.
























