




How Does Acetate Form and Why Is It Important in Chemistry?
Most of us are familiar with vinegar, it is a common kitchen ingredient having a sour taste. Vinegar is an aqueous solution of acetic acid (IUPAC name: ethanoic acid), with the chemical formula CH3COOH. When the acid loses a proton, the anion of the acetic acid is termed an ‘acetate anion’. When the acetate anion combines with a cation, the compound is called an ‘acetate’. The simplest acetate is hydrogen acetate, which is another name for acetic acid.
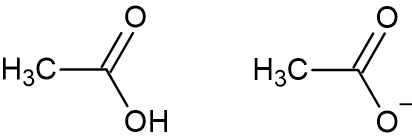
a) Acetic Acid b) Acetate Anion
Acetate is the conjugate base of acetic acid. The acetate ion is commonly abbreviated as AcO- or -OAc. Acetic acid is a weak acid which dissociates in water to release a proton and an acetate ion at a pH of 5.5 or above. Acetate chemical formula is CH3COO-
CH3COOH + H2O ⇌ CH3COO- + H3O+
A very well-known reaction of vinegar (acetic acid) and baking soda (sodium bicarbonate) produces an acetate ester (sodium acetate). This mixture is often used for cleaning purposes.
CH3COOH + NaHCO3 → CH3COONa + CO2 + H2O
In human biology, acetate is one of the most common building blocks for biosynthesis, such as fatty acid synthesis. It is a common anion found in Biology.
Table: Properties of Acetate Ion
Acetate Structure
Acetate contains one methyl group(-CH3) which is bonded with a carbonyl carbon. The carbonyl group is connected to another oxygen with a negative charge along with the methyl group.
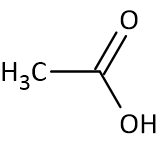
Acetate Anion
The methyl carbon is sp3 hybridised and has a tetrahedral geometry; the connected carbonyl carbon is sp2 hybridised that is doubly bonded to neutral oxygen and singly bonded to a negatively charged oxygen. However, both the oxygen is equivalent due to the delocalization of electrons through resonance.
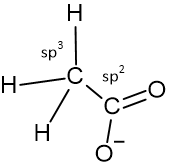
Hybridisation Sates of Acetate Anion
The negative charge over one oxygen shifts to form a double bond with the carbonyl carbon, while the existing double bond dissolves and the electron cloud is pushed over the other oxygen atom with the accumulation of negative charge. This delocalization results in equal distribution of the negative charge on both the oxygen atoms of the acetate anion.
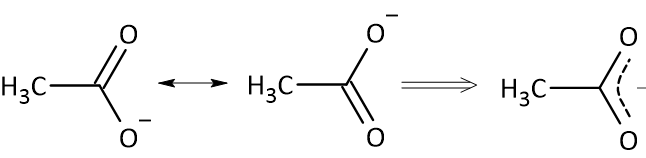
Delocalisation of Negative Charge by Resonance
Acetate Esters
In Organic Chemistry, acetate esters are the esters of acetic acid, where the acetate anion combines with an organyl group (R). Acetate esters are generally represented as CH3COOR. These esters are generally fragrant liquids with low toxicity and are lipophilic and, sometimes, volatile.
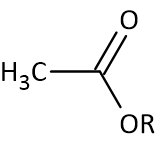
Acetate Ester
Acetate Biochemistry
Acetate is broken down in nature by fermentation carried out by methanogenic archaea. The microbe catalyses the dismutation of acetic acid, which is a disproportion reaction; a single electron from carbonyl (e- donor) of the carboxylic acid is transferred to the methyl group (e- acceptor), releasing methane and carbon dioxide.
CH3COO- + H+ → CH4 + CO2
Acetate is produced by gut bacteria by fermentation. It is also derived from diet and generated from various tissues like the intestine and liver. Deacylation reaction produces acetate in all cells. The acetates, in order to be utilised, are converted into acetyl coenzyme A (acetyl-CoA).
Acetyl-coenzyme A is converted into acetate while producing ATP (the energy currency) during substrate-level phosphorylation in E.Coli.
Acetyl-CoA + phosphate → acetyl-phosphate + CoA
Acetyl-phosphate + ADP → acetate + ATP
Acetyl CoA is involved in lipid, protein and carbohydrate metabolism. In the citric acid cycle, it delivers the acetyl group that leads to the production of energy.
Uses of Acetate
Acetate is used largely in the production of vinyl acetate, a precursor to polyvinyl alcohol used as a component of paints.
‘Acetate’ is often used as a substituent for cellulose acetate, which is produced using acetic acid. Cellulose acetate is used in the production of fibres.
Variety of industrial solvents are acetates, such as methyl acetate, ethyl acetate, isopropyl acetate and butyl acetate (used as a fragrance in food products).
Interesting Facts
Acetates have been found to have immunomodulatory properties, affecting innate immune response to pathogenic bacteria.
Most of the acetic acid produced annually (about 5 billion kilograms) is consumed in the production of acetates.
Key Features
‘Acetate’ refers to the compound containing the acetate anion and another cation.
Chemical formula of acetate anion is C2H3O2- or CH3COO-.
Acetic acid is the simplest acetate. Hydrogen cation and the acetate anion combine to form hydrogen acetate (acetic acid: CH3COOH)
Acetate is utilised in the form of acetyl CoA in biological systems. It is used in various metabolism to yield energy, and in biosynthesis, it serves as a common building block.
FAQs on Acetate in Chemistry: Meaning, Structure & Key Properties
1. What is acetate and what is its chemical formula?
Acetate is an anion, which is the conjugate base of acetic acid. It is formed when acetic acid loses a proton. The chemical formula for the acetate ion is CH₃COO⁻ or C₂H₃O₂⁻. A compound that contains this ion, combined with a cation (like sodium acetate, NaCH₃COO), is also referred to as an acetate.
2. What is the difference between acetate and acetic acid?
The primary difference lies in their chemical nature and charge. Acetic acid (CH₃COOH) is a neutral molecule and a weak acid. In contrast, the acetate ion (CH₃COO⁻) is its conjugate base, carrying a negative charge. Acetic acid can donate a proton to become acetate, while acetate can accept a proton to become acetic acid.
3. What are some common uses of acetate in industry and daily life?
Acetate has a wide range of applications based on its chemical form. Key uses include:
- Solvents: Acetate esters like ethyl acetate and butyl acetate are widely used as industrial solvents in paints, inks, and adhesives.
- Polymers: It is a key component in producing vinyl acetate, which is used to make polyvinyl acetate (PVA) glue and polyvinyl alcohol (a component in paints).
- Fabrics: Cellulose acetate, derived from wood pulp, is used to create a silky, wrinkle-resistant fabric also known as 'acetate'.
- Consumer Products: Cellulose acetate is also used to make products like eyeglass frames and photographic film.
4. What is an acetate ester and how is it formed?
An acetate ester is an organic compound with the general formula CH₃COOR, where 'R' is an organyl group (an alkyl or aryl group). These esters are formed through a reaction called esterification, typically by reacting acetic acid (CH₃COOH) with an alcohol (R-OH) in the presence of an acid catalyst. They are often known for their characteristic fruity or pleasant smells.
5. What is an acetate buffer and why is it used?
An acetate buffer is a solution that resists changes in pH. It is typically made by mixing a weak acid, acetic acid (CH₃COOH), with its conjugate base, usually in the form of a salt like sodium acetate (CH₃COONa). This buffer is effective in maintaining a stable pH in the range of approximately 3.5 to 5.5, making it useful in biochemical reactions and chemical processes that require a stable, acidic environment.
6. Why is the acetate ion considered a base?
The acetate ion (CH₃COO⁻) is considered a base because it is the conjugate base of a weak acid (acetic acid). According to the Brønsted-Lowry definition, a base is a substance that can accept a proton (H⁺). When dissolved in water, the acetate ion reacts with water in a hydrolysis reaction, accepting a proton from a water molecule to form acetic acid and hydroxide ions (OH⁻). This increase in the concentration of OH⁻ ions makes the solution basic.
7. How does resonance contribute to the stability of the acetate ion?
Resonance significantly stabilises the acetate ion by delocalising the negative charge. In the CH₃COO⁻ structure, the negative charge is not located on a single oxygen atom. Instead, it is spread equally across both oxygen atoms through resonance. This delocalisation means each carbon-oxygen bond has a character that is intermediate between a single and a double bond, making the ion more stable than if the charge were fixed on just one oxygen atom.
8. What is the role of acetate in biological systems like the human body?
In biology, acetate is a crucial metabolic intermediate. It is utilised in the form of acetyl coenzyme A (acetyl-CoA). This molecule is a central hub in metabolism, connecting the breakdown of carbohydrates, fats, and proteins. Acetyl-CoA enters the citric acid cycle (Krebs cycle) to generate ATP (energy), and it also serves as a fundamental building block for the biosynthesis of fatty acids and cholesterol.
9. Why are acetate esters often fragrant while acetic acid has a pungent smell?
The difference in smell arises from their molecular structure and volatility. Acetic acid is a small, polar molecule whose volatility allows it to be easily detected as a sharp, pungent vinegar smell. In contrast, acetate esters are generally less polar and more volatile. Their specific molecular shapes interact with olfactory receptors in our nose in a way that is perceived as pleasant, fruity, or sweet smells, making them common in perfumes and food flavourings.
10. Can acetate exist in a neutral state?
No, the term 'acetate' by itself specifically refers to the negatively charged ion (CH₃COO⁻). It cannot exist as a stable, neutral, isolated molecule. To become electrically neutral, the acetate ion must bond with a positive ion (cation). For example, it combines with a hydrogen ion (H⁺) to form the neutral molecule acetic acid (CH₃COOH), or with a sodium ion (Na⁺) to form the neutral salt sodium acetate (CH₃COONa).























