




How to Perform and Ace Salt Analysis in CBSE Class 12 Chemistry 2025-26
Analytical chemistry is the chemical analysis of a particular compound, which includes both qualitative and quantitative analysis of a substance. Qualitative analysis of inorganic salts is a process of detection of acidic and basic radicals present in a given salt. Inorganic salts are obtained through the complete or partial neutralisation of an acid and base.
Table of Contents
Aim
Apparatus Required
Theory: Dry Heating Test
Procedure
Observation
Result
Aim
To analyse the given salt for acidic and basic radicals using a dry heating test.
Apparatus required
Test tubes
Test tube holder
Salt sample
Bunsen burner
Theory
Qualitative analysis of various inorganic salts is based on two basic principles, i.e. Solubility of a product and the Common ion effect.
Solubility product is defined as the equilibrium present between a solid compound and its ions in a solution. Hence, when the ionic product of a salt exceeds the solubility product, precipitation of that particular ion takes place.
The common ion effect is what controls the ionic products of various salts. It is known to suppress the ionisation of a particular electrolyte when another electrolyte having a common ion is added to the solution.
Dry heating is a test which is used to detect anions and cations of a particular salt. The heating of salts causes them to undergo decomposition which leads to the release of a particular gas, or it may show a significant colour change in the residue.
Procedure
Take 0.1g of dry salt in a properly dry and clean test tube.
Heat the contents of the test tube over a Bunsen burner for about one minute.
Observe the changes occurring in the test tube.
Note down the observations.
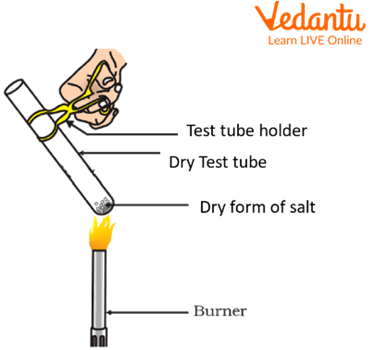
Dry heating test
Observations
Experiment | Observation | Inference |
Dry Heating Test: Heated a pinch of the salt in a dry test tube and noted the following observations: | ||
Gas Evolved | Reddish brown gas was released, turning the FeSO4 solution black. | NO3- may be present |
Sublimation | No sublimate is formed | Ammonium halides, aluminium chloride, and iodide may be absent. |
Decrepitation | No crackling sound is observed. | Lead nitrate, and barium nitrate, may be absent. |
Colour of the Residue | White | Ba2+, Sr2+, Ca2+, Al3+, Mg2+, etc., may be present. |
Result
To identify the acid and basic radicals in the given sample, a dry heating test is conducted, and it will help to identify nitrate, halides, copper, ferrous, zinc and cobalt ions.
Precautions
Use a completely dry test tube for the above test, not even a drop of water should be present.
While heating the test tube, do not heat it constantly, but keep it over an angle and heat it slowly by removing it to and fro from the flame.
Keep the test tube away from the mouth, nose and body.
Use all lab safety gadgets such as lab coats and hand gloves.
Use a proper, stable test tube holder to heat the test tube.
Lab Manual Questions
1. What are the basic steps involved in inorganic salt analysis?
Ans: For a systematic analysis of a given inorganic salt, the following steps are performed:-
Preliminary tests are conducted for the given salt. These are generally dry tests and hence use the powdered or dry form of the salt.
Determination of cations is conducted using a wet test followed by confirmatory tests for the derived action.
Determination of anions is conducted using a wet test, followed by confirmatory tests for the derived anion.
Both of the wet tests are conducted by using the solution of the salt.
2. What does a physical examination of salt mean?
Ans: Physical examination of salt is the study of the given salt for its colour, smell, density etc. This basic information helps us understand which ions might be present or absent in the given salt. E.g. If the colour of the salt is white then there is a possibility that the salt does not contain Cu2+, Mn2+, Co2+, Fe3+ etc ions.
3. What is a flame test?
Ans: Salts when reacted upon by conc. HCl from chlorides, which are volatile and their vapours, impart distinctive colour to the flame. In a flame test, a paste of salt and conc. HCl is prepared. With the help of a platinum wire, this salt paste is introduced in the non-luminous flame and colour change is observed. This test can determine the presence of some basic radicals such as Sr2+, Ba2+, Ca2+ etc.
4. What is aqua-regia?
Ans: Aqua-regia is a mixture of concentrated HCl and concentrated HNO3. It is prepared in a ratio of 3:1 and is used for dissolving salts which are insoluble in water, dil HCl and dil HNO3. A salt which does not dissolve in aqua regia is also termed an insoluble salt.
Viva Questions
1. How is O.S. made?
Ans: O.S is known as the original solution and is prepared by dissolving a small amount of the given salt into either water, dil HCl, dil HNO3 or aqua regia.
2. Give the uses of table salt.
Ans: Table salt is majorly used for flavouring food items and the preservation of food items. It is also used for tanning, dyeing, bleaching, etc.
3. Define normal salts.
Ans: Salts which are formed by the complete replacement of the ionizable H+ ions or OH- ions by metallic ions of a base are known as normal salts. E.g.- HCl reacts with NaOH giving NaCl and water.
4. Enlist the members present in group 4 basic radicals.
Ans: Group 4 of the basic radicals contain- Co2+, Mn2+, Ni2+, Zn2+
5. What does a radical group mean?
Ans: Radicals are also known as free radicals, and these are molecules which contain at least one unpaired electron.
6. Give the group reagent used for the detection of group 4 cations.
Ans: The group reagent for Group 4 cations is Hydrogen sulphide(H2S) in the presence of Ammonium hydroxide(NH4OH).
7. What are acids and bases?
Ans: Acid is a substance which is sour and turns blue litmus paper red. For, e.g.- HCl, and HNO3. The base is soapy and bitter to taste and turns red litmus paper blue. E.g.- NaOH, KOH, Ca(OH)2 etc.
8. How is salt formed?
Ans: Salt is formed due to the neutralisation reaction between an acid and base, and hence it generally has a neutral pH.
9. What is the general scale of acids and bases on a pH scale?
Ans: Acids have a pH range of 0-6 and bases have a pH range of 8-14. The pH 7 is for neutral compounds.
10. What is a double salt?
Ans: A salt formed by the crystallisation of two simple salts or from a mixture of their respective saturated salt solutions is known as double salt. E.g. Potash alum.
Practical Based Questions (MCQs)
Find the odd one out
Flame test
Borax bead test
Charcoal cavity test
Basic radicals group-III tests
Ans: Basic radicals group-III tests
Non-luminous flames are also known as____
Oxidising flame
Reducing flame
Medium flame
Green colour flame
Ans: Oxidising flame
The original solution is used for
Dry tests
Flame tests
Wet tests
Colour test
Ans: Wet tests
In salt analysis, cation is _____and anion is_____
Acidic radical, basic radical
Basic radical, acidic radical
Negative, positive
Both A and C
Ans: Basic radical, acidic radical
A salt which is formed from the partial replacement of hydroxyl groups from a polyacidic base by an acidic radical is a ____
Acidic salts
Basic salts
Normal salts
Mixed salt
Ans: Basic salt
A basic radicals list includes
Sodium, Ferrous, Gold, and Nickel ions
Sodium, Chlorine, Potassium, and Aluminium ions
Ferrous, Sulphate, Cobalt, and Mercury ions
Nitrate, Nitrite, Helium, and Chromium ions
Ans: Sodium, Ferrous, Gold, Nickel ions
Mention the group reagent of group I of cations
Dil sulphuric acid
Dil hydrochloric acid
Ammonium hydroxide
Hydrogen sulphide
Ans: Dil hydrochloric acid
Group III of cations contains___and ____
Magnesium ions, calcium ions
Lead ions, Copper ions
Magnesium ions, aluminium ions
Ferric ions, aluminium ions
Ans: Ferric ions, aluminium ions
Assertion: Acidic radical is negative in charge
Reason 1: It is formed due to the release of a negative charge from a base.
Reason 2: It is formed due to the release of hydrogen ions from an acid.
Both reasons are the correct explanation for the assertion
Both reasons are incorrect explanations for the assertion
Reason 1 is correct and Reason 2 is incorrect
Reason 2 is correct and Reason 1 is incorrect
Ans: Reason 2 is correct and Reason 1 is incorrect
State which of the following is true.
Anion comes from a base
Cation comes from an acid
Acid is a substance which donates a proton.
The base is a substance which donates a neutron.
Ans: Acid is a substance which donates a proton.
Conclusion
A dry test is one of the preliminary tests which is performed to identify the acidic and basic radicals present in salt. Preliminary tests do not indicate the exact radicals of salt, rather they are the probable ions which might be present in the salt.
FAQs on Step-by-Step Guide: Analyzing Acidic and Basic Radicals in Salts
1. What are the essential preliminary tests conducted for the qualitative analysis of an unknown salt as per the CBSE Class 12 curriculum?
For the qualitative analysis of an unknown inorganic salt, the essential preliminary tests are conducted on the dry salt sample before preparing a solution. These tests provide important clues about the possible presence or absence of certain radicals. The key tests include:
- Physical Examination: Noting the colour and smell of the salt. For instance, a blue salt may indicate the presence of Cu²⁺ ions.
- Dry Heating Test: Heating a small amount of the salt in a dry test tube to observe any colour changes, decrepitation, or evolution of gases.
- Flame Test: Making a paste of the salt with concentrated HCl and introducing it to a non-luminous flame to check for characteristic colours, which indicate radicals like Ca²⁺, Sr²⁺, or Ba²⁺.
- Charcoal Cavity Test: Used for identifying certain metallic radicals by heating the salt mixed with sodium carbonate in a charcoal cavity.
2. What is the difference between an acidic radical and a basic radical in salt analysis?
In the context of inorganic salt analysis, the two components of a salt are referred to as acidic and basic radicals.
- An acidic radical is the negatively charged ion (anion) that originates from an acid. For example, in sodium chloride (NaCl), the chloride ion (Cl⁻) is the acidic radical, derived from hydrochloric acid (HCl).
- A basic radical is the positively charged ion (cation) that originates from a base. In NaCl, the sodium ion (Na⁺) is the basic radical, derived from the base sodium hydroxide (NaOH).
The main objective of salt analysis is to systematically identify these two types of radicals.
3. How is an 'Original Solution' (O.S.) prepared for the wet tests in salt analysis, and what is the correct order of solvents to use?
An 'Original Solution' or O.S. is a clear solution of the given salt used for all wet tests, especially for identifying cations. It must be prepared systematically by trying to dissolve the salt in solvents in a specific order:
- Cold Distilled Water: First, try to dissolve a small amount of the salt in cold distilled water.
- Hot Distilled Water: If it does not dissolve in cold water, heat the mixture gently.
- Dilute Hydrochloric Acid (HCl): If the salt is insoluble in water, try dissolving it in dilute HCl.
- Concentrated Hydrochloric Acid (HCl): If it is still insoluble, use concentrated HCl.
It is crucial to follow this order because using a stronger solvent unnecessarily can interfere with subsequent tests for different groups of radicals.
4. Explain the principle of the common ion effect and its importance in the separation of Group II and Group IV basic radicals.
The common ion effect is the suppression of the dissociation of a weak electrolyte by the addition of a strong electrolyte containing a common ion. This principle is fundamental to the systematic separation of basic radicals.
- For Group II: The group reagent is H₂S gas passed through the solution acidified with dilute HCl. The HCl provides a high concentration of H⁺ ions (common ion), which suppresses the ionisation of the weak electrolyte H₂S. This results in a very low concentration of sulphide ions (S²⁻), which is just enough to precipitate the Group II cations (like Cu²⁺, Pb²⁺) that have low solubility products, but not the higher group cations.
- For Group IV: The group reagent is H₂S gas in an ammoniacal solution (NH₄OH). The NH₄OH provides OH⁻ ions, which remove H⁺ ions from the solution, shifting the H₂S equilibrium to the right and increasing the concentration of S²⁻ ions. This higher concentration is sufficient to precipitate Group IV cations (like Zn²⁺, Ni²⁺) which have higher solubility products.
5. What are group reagents in cation analysis, and what is the specific group reagent for identifying Group III basic radicals?
A group reagent is a specific chemical substance that can selectively precipitate a particular group of cations from a solution under certain conditions. This allows for the systematic separation and identification of basic radicals group by group.
For Group III basic radicals (like Al³⁺ and Fe³⁺), the group reagent is ammonium hydroxide (NH₄OH) in the presence of ammonium chloride (NH₄Cl). NH₄Cl is added first to suppress the ionisation of the weak base NH₄OH (due to the common ion effect of NH₄⁺), ensuring that the concentration of hydroxide ions (OH⁻) is just high enough to precipitate the hydroxides of Group III cations, but not those of subsequent groups.
6. Why is nitric acid (HNO₃) generally avoided when preparing the Original Solution for testing acidic radicals like chloride (Cl⁻)?
Using nitric acid (HNO₃) to prepare the Original Solution is avoided when testing for halide radicals (Cl⁻, Br⁻, I⁻) for a critical reason. Nitric acid is a strong oxidising agent. If halide ions are present, the nitric acid can oxidise them into their respective halogens (Cl₂, Br₂, I₂). This would interfere with the confirmatory tests for these radicals, such as the silver nitrate test, leading to incorrect or inconclusive results. Therefore, dilute HCl is a preferred acidic solvent if the salt is insoluble in water.
7. For the 2025-26 CBSE board practicals, what key precautions should a student take to ensure accurate results in a salt analysis experiment?
To ensure accuracy and safety during the salt analysis practical, students must observe the following precautions:
- Use Clean Apparatus: All test tubes, beakers, and glass rods must be thoroughly cleaned with distilled water to prevent contamination from previous experiments.
- Use Small Quantities: Use only a small amount of salt and reagents for each test. This not only conserves chemicals but also makes the reaction observations clearer.
- Follow the Correct Sequence: Tests for cation groups must be performed in the correct order (Group 0, I, II, III, etc.), as the presence of higher group ions can interfere with tests for lower groups.
- Handle Acids with Care: Always add acid slowly and cautiously, especially concentrated acids, and never point the mouth of a heating test tube towards yourself or others.
- Perform Confirmatory Tests: A preliminary test only suggests the possibility of a radical. Always perform a specific confirmatory test to confirm its presence beyond doubt.
8. How would you systematically identify the acidic and basic radicals in an unknown salt given to you as ammonium carbonate ((NH₄)₂CO₃)?
To analyse ammonium carbonate ((NH₄)₂CO₃), you would proceed as follows:
Analysis of Acidic Radical (CO₃²⁻):
- Preliminary Test: Take a pinch of salt in a test tube and add a few drops of dilute H₂SO₄. A brisk effervescence that turns lime water milky indicates the presence of the carbonate radical.
- Confirmatory Test: The lime water test (passing the evolved CO₂ gas through Ca(OH)₂ solution to form a white precipitate of CaCO₃) serves as the confirmation.
Analysis of Basic Radical (NH₄⁺):
- Group Analysis: Ammonium (NH₄⁺) belongs to Group 0, so it is tested first before other groups.
- Test: Take a small amount of the salt in a test tube and add sodium hydroxide (NaOH) solution. Heat the mixture gently. A characteristic pungent smell of ammonia gas is observed.
- Confirmatory Test: Bring a glass rod dipped in concentrated HCl near the mouth of the test tube. The formation of dense white fumes of ammonium chloride (NH₄Cl) confirms the presence of the ammonium radical.
























