Chemistry Notes for Chapter 11 The P Block Elements Class 11 - FREE PDF Download
In Cbse Class 11 Chemistry Notes Chapter 11 The P Block Elements, you’ll explore some important elements and their unique properties in the periodic table. This chapter teaches you about their trends, uses, and why they matter in daily life and exams. If the names and reactions feel confusing, don’t worry—these notes break it down step by step for you.
To prepare effectively, check the Class 11 Chemistry Syllabus for updated topics. Learning from clear notes means you spend less time stuck and more time understanding each concept.
Revision is simple with our Class 11 Chemistry Revision Notes on Vedantu. This chapter often appears in exams, so going over these notes is a smart way to feel confident and score better.
The p-Block Elements Class 11 Notes Chemistry - Basic Subjective Questions
Section – A (1 Mark Questions)
1. How does metallic and non-metallic character vary in a group?
Ans. The non-metals and the metals exist only in the p-block of the periodic table. The non-metallic character of elements decreases down the group. In fact the heaviest element in each p-block group is the most metallic in nature.
2. Third-period elements can expand their covalence above four. Briefly explain.
Ans. The third-period elements of p-groups included d-orbital, which can be utilized to form bond and expand octet.
3. Give two examples of electron deficient molecules.
Ans. BF3, Al2Cl6
4. Arrange the following halides of boron in the increasing order of acidic character: BF3, BCl3, BBr3, BI3.
Ans. BF3 < BCl3 < BBr3 < BI3
Since in BF3 there is back -bonding due to which it is less acidic among all .
5. Why CCl4 behaves as an electron precise molecule?
Ans. Carbon in CCl4, the number of electrons around the central atom in a molecule is eight and thus is electron precise molecule.
6. Lead unaffected by water. Why?
Ans. Lead is unaffected by water, probable because of a protective oxide film formation.
7. Why Diamond is the hardest substance known?
Ans. Diamond is the hardest substance on the earth because it is very difficult to break extended covalent bonding.
8. What is water gas composed of ?
Ans. The mixture of CO and H2 is known as water gas or synthesis gas.
9. What is the maximum covalence shown by N?
Ans. Nitrogen shows a maximum covalence of +4 because only four orbitals, one S and three P-orbitals are available for bonding in Nitrogen.
10. Bi(V) is a stronger oxidizing agent than Bi(III). Why?
Ans. Bi is more stable in +3 oxidation state in comparison to +5 due to inert pair effect. Therefore, Bi(V) has a strong tendency to act as oxidizing agent.
Section – B (2 Marks Questions)
11. SiCl4 forms [SiCl6]2– while CCl4 does not form [CCl6]2– Explain.
Ans. Carbon does not have d-orbitals and hence CCl4 does not combine with Cl– ions to give [CCl6]2–. On the other hand, silicon has vacant 3d-orbitals and thus can expand its covalency from 4 to 6. Therefore SiCl4 combines with Cl ions to form [SiCl6]2–.
12. Why does carbon form covalent compounds whereas lead forms ionic compounds?
Ans. Carbon cannot lose electrons to form C4 because the sum of four ionization enthalpies is very high. It cannot gain four electrons to form C4 because energetically it is not favourable. Hence, C forms only covalent compounds. Down the group 14, ionization enthalpies decrease, Pb being the last element has so low I.E. that it can lose electrons to form ionic compounds.
13. What are halides of carbon? Give few examples.
Ans. Carbon combines with halogens to form both simple and mixed tetrahalides. In the case of simple halides, all the four expected tetrahalides (e.g. CF4, CCl4, CBr4 and CCl4) are known to exist. The stability of the simple tetrahalides decreases with the increasing atomic mass of the halogen.
(CF4 > CCl4 > CBr4 > Cl4)
Amongst the mixed halides the better-known compounds are (CFCl3, CF2Cl2 and CCl3Br).
14. Why are boron halides & diborane referred to as “Electron deficient compounds”?
Ans. Boron in its halides has only six electrons in its valence shell, therefore it is short by two electrons to complete its octet. As a result, a molecule of boron halide can accept a pair of electrons from any electron-rich compound that is why boron halides are called electron-deficient compounds. In diborane, the total no. of valence electrons is not sufficient to completely fill the available orbitals. This gives an electron-deficient character to diborane.
15. All the elements of group 13 except thallium show a +3 oxidation state while it, shows a +1 oxidation state. Give reasons.
Ans. The valence shells of group 13-elements have two electrons in s-subshell & 1-electron in p-subshell. Therefore, they are expected to show a +3-oxidation state. In thallium, the electrons of the s-subshell do not take part in bond formation due to the inert pair effect & only one electron of the p-orbital participates in bond formation, thus it shows +1 oxidation state only.
16. Carbon different from other member of the group. Why?
Ans. Carbon differs from rest of the members of its group due to its smaller size, higher electro negativity, higher ionization enthalpy and unavailability of d-orbitals.
17. Covalence of carbon not expand beyond four. Explain briefly.
Ans. In carbon, only s and p orbitals are available for bonding and therefore, it can accommodate only four pairs of electrons around it. This limit the maximum covalence to four whereas other members can expand their covalence due to the presence of d-orbitals.
18. Boron forms no compounds in a unipositive state but thallium in a unipositive state is quite unstable. Why?
Ans. Boron has electronic configuration 2s22p1 & therefore forms compounds in a trivalent state. However, thallium prefers to form compounds in the +1 oxidation state rather than in the +3 oxidation state as suggested by its group number. This is due to the inert pair effect. According to this effect, the 6s2 electrons in the case of heavy metals preferably do not take part in bonding.
19. Why Borazine is more reactive than benzene?
Ans. Both Borazine & Benzene are isoelectronic. In benzene C = C bonds are non-polar while N = B bonds in borazine are polar in nature due to the presence of a co-ordinate bond between N & B atoms. As a result, addition is quite frequent in borazine while it is less in benzene because of delocalisation of pi-electron charge.
20. Why does CO2 have a linear shape with no dipole moment?
Ans. In CO2 molecule carbon atom undergoes sp hybridization. Two sp hybridized orbital of carbon atom overlap with two p-orbital’s of oxygen atoms to make two sigma bonds while other two electrons of carbon atom are involved in $p\pi-p\pi$ bonding with oxygen atom. This results in its linear shape [with both C-O bond of equal length (115 pm)] with no dipole moment.
PDF Summary - Class 11 Chemistry The p-Block Elements Notes (Chapter 13)
Introduction:
The p-block is made up of elements in groups 13 through 18 of the periodic table. Metals, metalloids, and non-metals are all found in the p-block. The electrical configuration of the p-block components is that of a generic valence shell configuration of ${\text{n}}{{\text{s}}^2}{\text{n}}{{\text{p}}^{1 - 6}}$. Because of their tiny size, strong electronegativity, and lack of d-orbitals, the initial members of each group from $13 - 17$ of the p-block elements differ in many ways from the other members of their respective groups.
When compared to the other members of the same group, the first member of a group has a stronger capacity to establish \[p\pi - p\pi \] multiple connections between itself (e.g. ${\text{C}} = {\text{C}},{\text{C}} \equiv $ ${\text{C}},{\text{N}} \equiv {\text{N}}$ ) and to elements in the second row$({\text{e}}.{\text{g C}} = {\text{O}},{\text{C}} = {\text{N}},{\text{C}} \equiv {\text{N}},{\text{N}} = {\text{O}})$. The group number minus 10 is the maximum oxidation of a p-block element. Due to the inert pair effect, the oxidation state two less than the maximum group oxidation state becomes more stable in groups 13 to 16. (s-subshell electrons' unwillingness to engage in chemical bonding)
Trends in Properties of P-Block Elements:
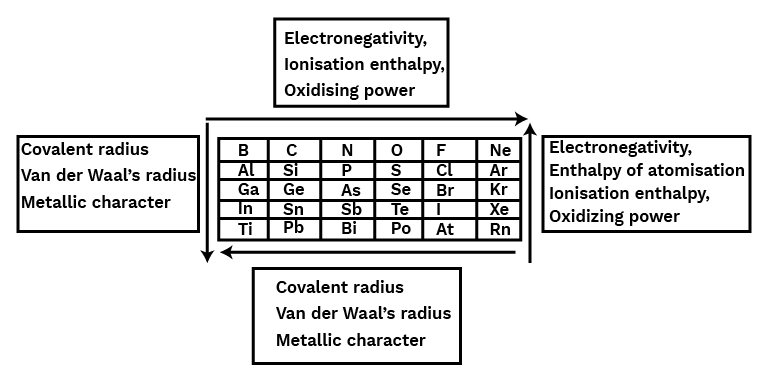
(A) Group 13 Elements: The Boron Family:
Boron is a non-metal, while aluminium is a metal with numerous chemical similarities to boron, while gallium, indium, and thallium are virtually entirely metallic.
Electronic Configuration:
The valence shell electronic configuration of elements of boron family is ${\text{n}}{{\text{s}}^2}{\text{n}}{{\text{p}}^1}$.
Atomic Radii:
As one moves along the group, an additional shell of electrons is added to each succeeding member, resulting in a rise in atomic radius. ${\text{Ga}}$ has a smaller atomic radius than ${\text{Al}}$. Because of the higher nuclear charge in gallium, the presence of an extra 10 d-electrons only provides a weak screening effect for the outer electrons. As a result, Gallium’s atomic radius( $135{\text{pm}}$ ) is less than that of Aluminium( $143{\text{pm}}$ ).
$\text{B}<\text{Ga}<\text{Al}<\text{In}<\text{Tl}$$\text{B}<\text{Ga}<\text{Al}<\text{In}<\text{Tl}$
Ionization Enthalpy:
The ionisation enthalpy values do not drop smoothly along the group as predicted from the overall patterns. The drop from ${\text{B}}$ to ${\text{Al}}$ is related with increases in size. The inability of ${\text{d and f }}$ electrons, which have a low screening effect, to compensate for the increase in nuclear charge is responsible for the observed discontinuity in ionisation enthalpy values between ${\text{Al}}$ and ${\text{Ga}}$, as well as between ${\text{In}}$ and ${\text{Tl}}$. The sum of each element's first three ionisation enthalpies is extremely high.
\[\text{B}>\text{Al}>\text{Ga}>\text{In}>\text{Tl}\]\[\text{B}>\text{Al}>\text{Ga}>\text{In}>\text{Tl}\]
Electronegativity:
Electronegativity falls from ${\text{B}}$ to ${\text{Al}}$ and then increases somewhat as you move down the group. This is due to differences in atomic size across the elements.
$\text{B}>\text{Tl}>\text{In}>\text{Ga}>\text{Al}$$\>\text{Tl}>\text{In}>\text{Ga}>\text{Al}$
Physical Properties:
Boron is a non-metallic element. It's a solid black colour that's incredibly hard. It comes in a variety of allotropic forms. Boron has an extraordinarily high melting point due to its extremely strong crystalline lattice. Soft metals having a low melting point and good electrical conductivity make up the rest of the group. Gallium, which has a low melting point( $303\;{\text{K}}$ ), might be liquid during the summer. Because of its high boiling point $(2676\;{\text{K}})$, it may be used to measure high temperatures. From boron through thallium, the density of the elements rises.
Chemical Properties:
Oxidation State and Trends in Chemical Reactivity:
The sum of boron's first three ionisation enthalpies is quite high due to its tiny size. This prohibits it from producing $ + 3$ ions, forcing it to exclusively produce covalent compounds. However, as we progress from ${\text{B}}$ to ${\text{Al}}$, the sum of the first three ionisation enthalpies of ${\text{Al}}$ falls significantly, allowing ${\text{Al}}$ to form ${\text{A}}{{\text{l}}^{ + 3}}$ ions. Effective nuclear charge, on the other hand, binds ns electrons closely down the group due to inadequate shielding, limiting their participation in bonding. As a result, bonding may include solely the p-orbital electron. In reality, both $ + 1$ and $ + 3$ oxidation states have been reported in ${\text{Ga, In and Tl}}$. For heavier elements, the relative stability of the $ + 1$oxidation state increases: ${\text{Al}} < {\text{Ga}} < \ln < {\text{TI}}$. The $ + 1$ oxidation state is the most common in thallium, with the $ + 3$oxidation state being the most oxidising. Energy considerations predict that compounds in the $ + 1$ oxidation state are more ionic than those in the $ + 3$oxidation state. The number of electrons surrounding the central atom in a molecule of these elements' compounds (for example, boron in ${\text{B}}{{\text{F}}_{\text{3}}}$) will be just six in the trivalent state.
These electron-deficient molecules have a proclivity to take a pair of electrons in order to reach a stable electronic state, and so act as Lewis acids. The inclination to act as Lewis acid reduces as the size of the group grows smaller. To create ${\text{BC}}{{\text{l}}_{\text{3}}}{\text{.N}}{{\text{H}}_{\text{3}}}$, ${\text{BC}}{{\text{l}}_{\text{3}}}$simply obtains a lone pair of electrons from ammonia. Most covalent compounds are hydrolysed in water when they are in a trivalent state. The trichloride forms a tetrahedral species ${\left[ {{\text{M}}{{({\text{OH}})}_4}} \right]^ - }$when it is hydrolysed in water; aluminium chloride forms an octahedral ion ${\left[ {{\text{Al}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{3 + }}$ in an acidified aqueous solution.
Reactivity Towards Air:
Boron, in its crystalline state, is non-reactive. On the surface of aluminium, a very thin oxide layer develops, protecting the metal from further assault. ${{\text{B}}_2}{{\text{O}}_3}$ and ${\text{A}}{{\text{l}}_2}{{\text{O}}_3}$ are formed when amorphous boron and aluminium metal are heated in the air. Nitrides are created when dinitrogen is heated to a high temperature.
$2{\text{E}}({\text{s}}) + 3{{\text{O}}_2}(\;{\text{g}})\xrightarrow{\Delta }2{{\text{E}}_2}{{\text{O}}_3}(\;{\text{s}}){\text{ }};{\text{ }}2{\text{E}}({\text{s}}) + {{\text{N}}_2}(\;{\text{g}})\xrightarrow{\Delta }2{\text{EN}}({\text{s}})$
The character of these oxides changes as you move through the group. Boron trioxide is an acidic compound that forms metal borates when it combines with basic (metallic) oxides. The oxides of aluminium and gallium are amphoteric, while those of indium and thallium are basic.
Reactivity Towards Acids and Alkalies:
Even at moderate temperatures, boron does not react with acids and alkalies; nevertheless, aluminium dissolves in mineral acids and aqueous alkalies, giving it an amphoteric property. Dihydrogen is generated when aluminium is dissolved in dilute ${\text{HCl}}$. Concentrated nitric acid, on the other hand, makes aluminium inactive by creating a protective oxide coating on the surface. When aluminium interacts with aqueous alkali, it produces dihydrogen.
$\text{2Al(s)+6HCl(aq)}\xrightarrow{{}}\text{2A}{{\text{l}}^{\text{3 + }}}\text{(aq) + 6C}{{\text{l}}^{\text{ - }}}\text{ + 3}{{\text{H}}_{\text{2}}} $
$\text{2Al(s) + 2NaOH(aq) + 6}{{\text{H}}_{\text{2}}}\text{O(l) }\xrightarrow{{}}\text{2N}{{\text{a}}^{\text{ + }}}{{\left[ \text{Al(OH}{{\text{)}}_{\text{4}}} \right]}^{\text{ - }}}(\text{aq})\,\text{+}\,\text{3}{{\text{H}}_{\text{2}}}\text{(g)} $
$\text{sodium tetrahydroxoaluminate(III)} $
Reactivity Towards Halogens:
These elements react with halogen to form trihalides (except ${\text{Tl}}{{\text{I}}_3}$ ). $2{\text{E}}({\text{s}}) + 3{{\text{X}}_2}(\;{\text{g}}) \to 2{\text{E}}{{\text{X}}_3}(\;{\text{s}})\quad ({\text{X}} = {\text{F}},{\text{ClBr}},{\text{l}})$
Important Trends and Anomalous Properties of Boron:
All of these elements' tri-chlorides, bromides, and iodides, which are covalent in nature, are hydrolysed in water. Except for boron, species like tetrahedral ${\left[ {{\text{M}}{{({\text{OH}})}_4}} \right]^ - }$ and octahedral ${\left[ {{\text{M}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{3 + }}$ occur in aqueous medium. The highest covalence of boron is 4 owing to the absence of d orbitals. The highest covalence may be predicted beyond 4 since d-orbitals are accessible with Al and other elements.
Boron (B):
Occurrence:
Boron may be found in the following minerals in nature:
Borax ${\left( {{\text{N}}{{\text{a}}^ + }} \right)_2}\;{{\text{B}}_4}{\text{O}}_7^{2 - }.10{{\text{H}}_2}{\text{O}}.$ (Boron is part of an anionic complex),
Boric acid ${{\text{H}}_3}{\text{B}}{{\text{O}}_3}$,
Kernite ${\text{N}}{{\text{a}}_2}\;{{\text{B}}_4}{{\text{O}}_7} \cdot 4{{\text{H}}_2}{\text{O}}$
Colemanite ${\text{C}}{{\text{a}}_2}\;{{\text{B}}_6}{{\text{O}}_{11}} \cdot 5{{\text{H}}_2}{\text{O}}$
Extraction of Boron:
In the absence of oxygen, by reducing ${{\text{B}}_2}{{\text{O}}_3}$ with magnesium, sodium, or potassium:
${\text{N}}{{\text{a}}_2}\;{{\text{B}}_4}{{\text{O}}_7} + 2{\text{HCl}} + 5{{\text{H}}_2}{\text{O}} \to 4{{\text{H}}_3}{\text{B}}{{\text{O}}_3} + 2{\text{NaCl}}$
$2{{\text{H}}_3}{\text{B}}{{\text{O}}_3}\xrightarrow{\Delta }{{\text{B}}_2}{{\text{O}}_3} + 3{{\text{H}}_2}{\text{O }};{\text{ }}{{\text{B}}_2}{{\text{O}}_3} + 3{\text{Mg}}\xrightarrow{{}}2\;{\text{B}} + 3{\text{MgO}}$
The product is heated with ${\text{HCl}}$ and filtered after ${{\text{K}}_2}{\text{O}}$ or ${\text{MgO}}$ dissolves, leaving elemental boron behind. Before being dried, it is thoroughly washed to remove the ${\text{HCl}}$This technique produces a dark amorphous boron powder ${\text{B}}$.
By heating potassium fluoroborate$\left( {{\text{KB}}{{\text{F}}_4}} \right)$ with potassium metal, you can make boron.
${\text{KB}}{{\text{F}}_4} + 3\;{\text{K}}\xrightarrow{{}}4{\text{KF}} + {\text{B}}$
It is then treated with dilute ${\text{HCl}}$ to remove ${\text{KF}}$ and ${\text{B}}$ is then washed and dried.
${\text{B}}$ is then washed and dried after being treated with dilute${\text{HCl}}$ to eliminate ${\text{KF}}$.
In tiny amounts in pure form (crystalline boron) as a result of
Reduction of ${\text{BB}}{{\text{r}}_3}$with ${{\text{H}}_2}$on a heated titanium metal filament at $1275 - 1475\;{\text{K}}$. The vapours of ${\text{B}}{{\text{r}}_2}$are absorbed in${\text{Cu}}$, and the vapours of boron that remain are condensed.
Decomposition of ${\text{B}}{{\text{I}}_3}$vapours using tungsten electrodes and a high-tension arc ($80{\text{kV}}$ ).
$2{\text{B}}{{\text{I}}_3}\xrightarrow{{}}2\;{\text{B}} \uparrow + 3{{\text{I}}_2} \uparrow $ (VanArkel method).
Properties:
It comes in five different varieties, four of which are crystalline and one of which is amorphous. All crystalline forms are made up of clusters of ${{\text{B}}_{12}}$units and are extremely hard. All crystalline forms are chemically inert and appear black. Melting points are in the range of ${2300^\circ }{\text{C}}$. The amorphous form, on the other hand, is brown and chemically active.
Reaction With Air:
\[2{\text{E}}({\text{s}}) + 3{{\text{O}}_2}({\text{g}}){\mkern 1mu} {\mkern 1mu} {\mkern 1mu} \to \,{\mkern 1mu} 2{{\text{E}}_2}{{\text{O}}_3}({\text{s}});2{\text{E}}({\text{s}}) + {{\text{N}}_2}(\;{\text{g}}){\mkern 1mu} {\mkern 1mu} \to 2{\text{EN}}({\text{s}})\]
Action of Alkalies and Acids:
$2\;{\text{B}} + 2{\text{NaOH}} + 2{{\text{H}}_2}{\text{O}}\xrightarrow{{}}2{\text{NaB}}{{\text{O}}_2} + 3{{\text{H}}_2}$
${\text{2 B + 3}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\xrightarrow{{{\text{oxidation}}}}{\text{2}}{{\text{H}}_{\text{3}}}{\text{B}}{{\text{O}}_{\text{3}}}{\text{ + 3S}}{{\text{O}}_{\text{2}}}$
${\text{2B + 6HN}}{{\text{O}}_{\text{3}}}\xrightarrow{{{\text{oxidation}}}}{\text{2}}{{\text{H}}_{\text{3}}}{\text{B}}{{\text{O}}_{\text{3}}}{\text{ + 6N}}{{\text{O}}_{\text{2}}}$
Reaction with ${\text{Mg}}$ and ${\text{Ca}}$ :
$3{\text{Mg}} + 2\;{\text{B}}\xrightarrow{{}}{\text{M}}{{\text{g}}_3}\;{{\text{B}}_2}$
${\text{3Ca + 2B}}\xrightarrow{{}}{\text{C}}{{\text{a}}_{\text{3}}}{\text{}}{{\text{B}}_{\text{2}}}$
${\text{M}}{{\text{g}}_3}\;{{\text{B}}_2}$ on repetitive hydrolysis gives diborane.
${\text{M}}{{\text{g}}_{\text{3}}}{\;}{{\text{B}}_{\text{2}}}{\text{ + 6HCl}}\xrightarrow{{{\text{hydrolysis}}}}{\text{ 3MgC}}{{\text{l}}_{\text{2}}}{\text{ + }}{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{ ; }}{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{ + 6}}{{\text{H}}_{\text{2}}}{\text{O}}\xrightarrow{{}}{\text{2}}{{\text{H}}_{\text{3}}}{\text{B}}{{\text{O}}_{\text{3}}}{\text{ + 6}}{{\text{H}}_{\text{2}}}$
Reducing Properties:
${\text{3Si}}{{\text{O}}_{\text{2}}}{\text{ + 4 B}}\xrightarrow{{}}{2\;}{{\text{B}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{ + 3Si}}$
${\text{3C}}{{\text{O}}_{\text{2}}}{\text{ + 4 B}}\xrightarrow{{}}{2\;}{{\text{B}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{ + 3C}}$
It decays in steam liberating hydrogen gas.
${\text{2B + 3}}{{\text{H}}_{\text{2}}}{\text{O(steam)}}\xrightarrow{{}}{{\text{B}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{ + 3}}{{\text{H}}_{\text{2}}}$
Uses:
Boron is utilised in the manufacture of high-impact steel and, since it absorbs neutrons, in reactor rods for atomic reaction control.
Compounds Of Boron:
Boron Trioxide $\left( {{{\text{B}}_2}{{\text{O}}_3}} \right)$ :
Preparation:
${{\text{H}}_{\text{3}}}{\text{B}}{{\text{O}}_{\text{3}}}{\text{ }}\mathop{\xrightarrow{{{\text{10}}{{\text{0}}^\circ }{\text{C}}}}}\limits^{} {\text{ HB}}{{\text{O}}_{\text{2}}}{\text{ }}\mathop{\xrightarrow{{{\text{16}}{{\text{0}}^{\text{o }}}{\text{C}}}}}\limits^{}{\text{}}{{\text{H}}_{\text{2}}}{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{7}}}\xrightarrow{{{\text{red heat}}}}{\text{ }}{{\text{B}}_{\text{2}}}{{\text{O}}_{\text{3}}} $
$ {\text{boric acid}} $
Properties:
It is a slightly acidic oxide that forms borates when it interacts with alkalies or bases.$3{\text{N}}{{\text{a}}_2}{\text{O}} + {{\text{B}}_2}{{\text{O}}_3} \to 2{\text{N}}{{\text{a}}_3}{\text{B}}{{\text{O}}_3}$ (sodium orthoborate).
It forms orthoboric acid after a gradual reaction with water. It generates coloured compounds when heated with transition metal salts.${{\text{H}}_2}{\text{O}} + {{\text{B}}_2}{{\text{O}}_3} \to 2{\text{HB}}{{\text{O}}_2};\quad {\text{HB}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \to {{\text{H}}_3}{\text{B}}{{\text{O}}_3}$
${{\text{B}}_2}{{\text{O}}_3} + {{\text{P}}_2}{{\text{O}}_5} \rightleftharpoons 2{\text{BP}}{{\text{O}}_4}$
Orthoboric Acid $H_{3}BO_{3}$ :
Among the oxyacids of boron are
Preparation:
A concentrated solution of borax is treated with sulphuric acid to precipitate it.${\text{N}}{{\text{a}}_2}\;{{\text{B}}_4}{{\text{O}}_7} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4} + 5{{\text{H}}_2}{\text{O}} \to {\text{N}}{{\text{a}}_2}{\text{S}}{{\text{O}}_4} + 4{{\text{H}}_3}{\text{B}}{{\text{O}}_3} \downarrow $
${{\text{H}}_3}{\text{B}}{{\text{O}}_3}$ is made by suspending powdered colemanite in water and filtering the surplus ${\text{S}}{{\text{O}}_2}$. White crystals of ${{\text{H}}_3}{\text{B}}{{\text{O}}_3}$are produced after filtering and chilling the filtrate.
${\text{C}}{{\text{a}}_2}\;{{\text{B}}_6}{{\text{O}}_{11}} + 2{\text{S}}{{\text{O}}_2} + 11{{\text{H}}_2}{\text{O}}\xrightarrow{{}}2{\text{Ca}}{\left( {{\text{HS}}{{\text{O}}_3}} \right)_2} + 6{{\text{H}}_3}{\text{B}}{{\text{O}}_3}$
Properties:
It is a weak monobasic acid, and the boron atom completes its octet in aqueous solution by eliminating ${\text{O}}{{\text{H}}^ - }$ from water molecules:
${\text{B}}{({\text{OH}})_3}({\text{aq}}) + 2{{\text{H}}_2}{\text{O}}(\ell ) \to {\text{B}}({\text{OH}})_4^ - ({\text{aq}}) + {{\text{H}}_3}{{\text{O}}^ + }({\text{aq}})$
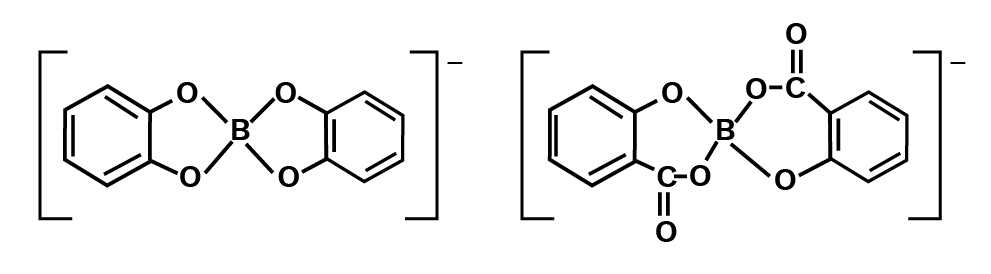
As a result, it acts as a Lewis acid rather than a proton donor. When a polyhydroxy molecule like glycol or glycerol is added to its aqueous solution, it behaves as a strong acid. The great stability of the conjugate bone chelate complex accounts for the acidity.
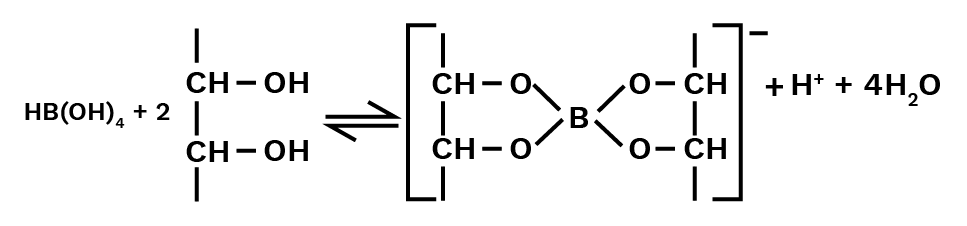
Catechol and salicylic acids create comparable complexes, but ethanol does not.
When heated, it produces metaboric acid $\left( {{\text{HB}}{{\text{O}}_2}} \right)$ first, followed by boron trioxide.
${{\text{H}}_{\text{3}}}{\text{B}}{{\text{O}}_{\text{3}}}{\text{ }}\mathop{\xrightarrow{{{\text{10}}{{\text{0}}^{\circ}}{\text{C}}}}}\limits^{} {\text{ HB}}{{\text{O}}_{\text{2}}}{\text{ }}\mathop {\xrightarrow{{{\text{16}}{{\text{0}}^{\circ }}{\text{C}}}}}\limits^{} {\text{}}{{\text{H}}_{\text{2}}}{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{7}}}\xrightarrow{{{\text{redheat}}}}{\text{ }}{{\text{B}}_{\text{2}}}{{\text{O}}_{\text{3}}} $
$ {\text{boric acid}} $
Orthoboric acid is oily to the touch and is less soluble in cold water than hot water. It has a multilayer structure with hydrogen bonds connecting planar units of ${\text{B}}{{\text{O}}_3}$.
Test For Borate Radical:
The evolved gas is burnt when boric acid is heated with ethyl alcohol, creating a green edged flame.
${{\text{H}}_{\text{3}}}{\text{B}}{{\text{O}}_{\text{3}}}{\text{ + 3}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}\xrightarrow{{}}{\text{B}}{\left({{\text{O}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}}\right)_{\text{3}}}{\text{ + 3}}{{\text{H}}_{\text{2}}}{\text{O}} $
$ {\text{ ethyl borate }}\left( {{\text{volatile}}} \right)$
Uses:
It's an antiseptic, and the water solution may be used to cleanse your eyes. In addition, it is utilised in the glass, enamel, and pottery industries.
Borax $\left( {{\mathbf{N}}{{\mathbf{a}}_{\mathbf{2}}}{{\text{B}}_4}{{\text{O}}_7} \cdot 10{{\text{H}}_2}{\text{O}}} \right):$
Preparation:
Borax can be found in nature, but it can also be produced using the methods listed below.
From Colemanite mineral. When colemanite powder is heated in a solution of ${\text{N}}{{\text{a}}_2}{\text{C}}{{\text{O}}_3}$, the precipitation of ${\text{CaC}}{{\text{O}}_3}$ takes place.
When white crystals of borax precipitate, the filterate is cooled. On treatment with ${\text{C}}{{\text{O}}_2}$, the mother liquor changes ${\text{NaB}}{{\text{O}}_2}$ to ${\text{N}}{{\text{a}}_2}{{\text{B}}_4}{{\text{O}}_7}$, which precipitates out after crystallisation.
${\text{4NaB}}{{\text{O}}_{\text{2}}}{\text{ + C}}{{\text{O}}_{\text{2}}}\xrightarrow{{}}{\text{N}}{{\text{a}}_{\text{2}}}{\text{\;}}{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{7}}}{\text{ + N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}$
From orthoboric acid. The action of \[{\text{N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\]on orthoboric acid produces borax.
${\text{4}}{{\text{H}}_{\text{3}}}{\text{B}}{{\text{O}}_{\text{3}}}{\text{ + N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\xrightarrow{{}}{\text{N}}{{\text{a}}_{\text{2}}}{\;}{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{7}}}{\text{ + 6}}{{\text{H}}_{\text{2}}}{\text{O + C}}{{\text{O}}_{\text{2}}} \uparrow $
Properties:
Borax is a white powder that is more soluble in hot water than cold water.
Because of its hydrolysis to weak acid ${{\text{H}}_{\text{3}}}{\text{B}}{{\text{O}}_{\text{3}}}$and strong alkali ${\text{NaOH}}$, its aqueous solution is alkaline.
${\text{N}}{{\text{a}}_{\text{2}}}\;{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{7}}}{\text{ + 7}}{{\text{H}}_{\text{2}}}{\text{O}}\xrightarrow{{}}{\text{4}}{{\text{H}}_{\text{3}}}{\text{B}}{{\text{O}}_{\text{3}}}{\text{ + 2NaOH}}$
When borax powder is heated, it expands at first owing to water loss in the form of steam, but then it transforms into a colourless clear borax bead at ${740^\circ }{\text{C}}$.
${{\text{N}}{{\text{a}}_{\text{2}}}\;{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{7}}} \times {\text{10}}{{\text{H}}_{\text{2}}}{\text{O}}\xrightarrow{\Delta }{\text{N}}{{\text{a}}_{\text{2}}}\;{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{7}}}{\text{ + 10}}{{\text{H}}_{\text{2}}}{\text{O}} \uparrow } $ ${{\text{N}}{{\text{a}}_{\text{2}}}\;{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{7}}}\xrightarrow{{{\text{74}}{{\text{0}}^{\text{o}}}{\text{C}}}}{\text{2NaB}}{{\text{O}}_{\text{2}}}{\text{ + }}{{\text{B}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{ (borax bead) }}}$
Action of Acids:
${\text{N}}{{\text{a}}_2}\;{{\text{B}}_4}{{\text{O}}_7} + 2{\text{HCl}} + 5{{\text{H}}_2}{\text{O}}\xrightarrow{{}}2{\text{NaCl}} + 4{{\text{H}}_3}{\text{B}}{{\text{O}}_3}{\text{ (boric acid) }}$
The white flakes of boric acid are produced by heating.\[{\text{N}}{{\text{a}}_{\text{2}}}{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{7}}}{\text{ }}\xrightarrow{{{\text{NaOH}}}}{\text{ NaB}}{{\text{O}}_{\text{2}}}\xrightarrow{{{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}}}{\text{ N}}{{\text{a}}_{\text{2}}}\left[ {{{{\text{(OH)}}}_{\text{2}}}{\text{ B(O - O}}{{\text{)}}_{\text{2}}}{\text{ B(OH}}{{\text{)}}_{\text{2}}}} \right]{\text{ 6}}{{\text{H}}_{\text{2}}}{\text{O}}\]
Correct formula of borax is ${\text{N}}{{\text{a}}_{\text{2}}}\left[ {{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{5}}}{{{\text{(OH)}}}_{\text{4}}}} \right]{\text{.8}}{{\text{H}}_{\text{2}}}{\text{O}}$
Bead Test: Boric anhydride reacts with certain metal salts such as, ${\text{N}}{{\text{i}}^{{\text{2 + }}}}{\text{, C}}{{\text{u}}^{{\text{2 + }}}}{\text{, C}}{{\text{o}}^{{\text{2 + }}}}{\text{, M}}{{\text{n}}^{{\text{2 + }}}}{\text{, C}}{{\text{r}}^{{\text{3 + }}}}$
To form coloured metaborates. The colour of the metaborates can be used to identify the metallic ions (cations) in salts.
${\text{N}}{{\text{a}}_{\text{2}}}{\;}{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{7}}}{\text{.10}}{{\text{H}}_{\text{2}}}{\text{O}}\mathop {\mathop {\xrightarrow[{{\text{ - 10}}{{\text{H}}_{\text{2}}}{\text{O}}}]{{\Delta}}}\limits^{} }\limits_{} {\text{N}}{{\text{a}}_{\text{2}}}{\;}{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{7}}}\xrightarrow{{{\text{74}}{{\text{0}}^{\text{o}}}{\text{C}}}}\underbrace {{\text{NaB}}{{\text{O}}_{\text{2}}}{\text{ + }}{{\text{B}}_{\text{2}}}{{\text{O}}_{\text{3}}}}_{{\text{glassy mass }}}{\text{ ; CuO + }}{{\text{B}}_{\text{2}}}{{\text{O}}_{\text{3}}}\xrightarrow{{}}{\text{Cu}}{\left( {{\text{B}}{{\text{O}}_{\text{2}}}} \right)_{\text{2}}}{\text{(blue bead)}}$
Uses: It is utilised in borax bead test, in gold purification, as flux during welding of metals and in glass production process.
Diborane $(B_{2}H_{6})$: Binary compounds of ${\text{B}}$ with ${\text{H}}$ are called boron hydrides or boranes. These compounds form following two types of series:
${{\text{B}}_{\text{n}}}{{\text{H}}_{{\text{n}} + 4}} - {{\text{B}}_2}{{\text{H}}_6},\;{{\text{B}}_5}{{\text{H}}_9},\;{{\text{B}}_6}{{\text{H}}_{10}},\;{{\text{B}}_{10}}{{\text{H}}_{14}}$
${{\text{B}}_{\text{n}}}{{\text{H}}_{{\text{n}} + 6}} - \quad {{\text{B}}_4}{{\text{H}}_{10}},\;{{\text{B}}_5}{{\text{H}}_{11}},\;{{\text{B}}_6}{{\text{H}}_{12}},\;{{\text{B}}_9}{{\text{H}}_{15}}$
The chemistry of diborane has piqued attention due to its use in a variety of synthetic processes, as well as the fact that the structure's elucidation aided in the clarification of fundamental ideas regarding the structure of electron deficient compounds.
Preparation:
${\text{4B}}{{\text{F}}_{\text{3}}}{\text{ + 3LiAl}}{{\text{H}}_{\text{4}}}\xrightarrow{{{\text{ether}}}}{\text{2}}\;{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{ + 3LiF + 3Al}}{{\text{F}}_{\text{3}}}$
${\text{2BC}}{{\text{l}}_{\text{3}}}{\text{ + 6}}{{\text{H}}_{\text{2}}}{\text{(excess)}}\xrightarrow{{{\text{silent electric discharge}}}}{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{ + 6HCl}}$
${\text{8B}}{{\text{F}}_{\text{3}}}{\text{ + 6LiH}}\xrightarrow{{{\text{ether}}}}{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{ + 6LiB}}{{\text{F}}_{\text{4}}}$
${\text{2NaB}}{{\text{H}}_{\text{4}}}{\text{ + }}{{\text{I}}_{\text{2}}}\xrightarrow{{{\text{ether}}}}{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{ + 2NaI + }}{{\text{H}}_{\text{2}}}$
${\text{3NaB}}{{\text{H}}_{\text{4}}}{\text{ + 4B}}{{\text{F}}_{\text{3}}}\xrightarrow[{{\text{450K}}}]{{{\text{ether}}}}{\text{3NaB}}{{\text{F}}_{\text{4}}}{\text{ + 2}}{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}$
It can also be obtained by addition of ${\text{NaB}}{{\text{H}}_4}$ to concentrated ${{\text{H}}_2}{\text{S}}{{\text{O}}_4}$ or ${{\text{H}}_3}{\text{P}}{{\text{O}}_4}$. ${\text{2NaB}}{{\text{H}}_{\text{4}}}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\xrightarrow{{}}{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{ + N}}{{\text{a}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$
$2{\text{NaB}}{{\text{H}}_4} + 2{{\text{H}}_3}{\text{P}}{{\text{O}}_4}\xrightarrow{{}}{{\text{B}}_2}{{\text{H}}_6} + 2{{\text{H}}_2} + 2{\text{Na}}{{\text{H}}_2}{\text{P}}{{\text{O}}_4}$
${\text{2B}}{{\text{F}}_{\text{3}}}{\text{ + 6NaH}}\xrightarrow{{{\text{450K}}}}{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{ + 6NaF}}$ (Industrial method)
Properties:
Diborane is a colourless gas having boiling point $183\;{\text{K}}$ which is briskly decomposed by water with the formation of ${{\text{H}}_{\text{3}}}{\text{B}}{{\text{O}}_{\text{3}}}{\&}{{\text{H}}_{\text{2}}}$ :
${{\text{B}}_2}{{\text{H}}_6} + 6{{\text{H}}_2}{\text{O}}\xrightarrow{{}}2{{\text{H}}_3}{\text{B}}{{\text{O}}_3} + 6{{\text{H}}_2}$
Diborane mixtures with air or oxygen spontaneously combust, releasing a substantial quantity of heat. Most other fuels have a lower heat of combustion per unit weight of fuel than diborane. As a result, it is utilised as a rocket fuel. ${{\text{B}}_2}{{\text{H}}_6} + 3{{\text{O}}_2}\xrightarrow{{}}{{\text{B}}_2}{{\text{O}}_3} + 3{{\text{H}}_2}{\text{O }}\Delta {\text{H}} = - 1976\;{\text{kJ}}\;{\text{mo}}{{\text{l}}^{ - 1}}$
Pyrolysis of diborane in sealed containers at temperatures above $375\;{\text{K}}$ is a complicated process that results in a mixture of boranes. eg, ${{\text{B}}_4}{{\text{H}}_{10}},\;{{\text{B}}_5}{{\text{H}}_9},\;{{\text{B}}_6}{{\text{H}}_{12}}$, and ${{\text{B}}_{10}}{{\text{H}}_{14}}$ .
Organoboranes are formed when diborane reacts easily with alkenes and alkynes in ether solvents at normal temperature. The hydroboration reaction is the name given to this process.
${{\text{B}}_2}{{\text{H}}_6} + {\text{HCl}}\xrightarrow{{}}{{\text{B}}_2}{{\text{H}}_5}{\text{Cl}} + {{\text{H}}_2}$
${{\text{B}}_2}{{\text{H}}_6} + 6{\text{MeOH}}\xrightarrow{{}}2\;{\text{B}}{({\text{OMe}})_3} + 6{{\text{H}}_2}$
Cleavage reactions
${{\text{B}}_2}{{\text{H}}_6} + 2{\text{M}}{{\text{e}}_3}\;{\text{N}}\xrightarrow{{}}2{\text{M}}{{\text{e}}_3}{\text{NB}}{{\text{H}}_3}$
${{\text{B}}_2}{{\text{H}}_6} + 2{\text{M}}{{\text{e}}_3}{\text{P}}\xrightarrow{{}}2{\text{M}}{{\text{e}}_3}{\text{PB}}{{\text{H}}_3}$
${{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{ + 2CO}}\xrightarrow{{{\text{20}}{{\text{0}}^{\text{o}}}{\text{C, 20atm}}}}{\text{2B}}{{\text{H}}_{\text{3}}}{\text{CO}}$ (borane carbonyl)
${{\text{B}}_2}{{\text{H}}_6} + 2{\text{E}}{{\text{t}}_2}\;{\text{S}}\xrightarrow{{}}2{\text{E}}{{\text{t}}_2}{\text{SB}}{{\text{H}}_3}$
$3\;{{\text{B}}_2}{{\text{H}}_6} + 6{\text{N}}{{\text{H}}_3}\xrightarrow{{{\text{low temprature}}}}{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{.2N}}{{\text{H}}_{\text{3}}}$ or ${\left[ {{\text{B}}{{\text{H}}_{\text{2}}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{2}}}} \right]^{\text{ + }}}{\text{BH}}_{\text{4}}^{\text{ - }}\xrightarrow{{{\text{20}}{{\text{0}}^{\text{o}}}{\text{C}}}}{{\text{B}}_{\text{3}}}{\;}{{\text{N}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{(borazole) + 12}}{{\text{H}}_{\text{2}}}$
${{\text{B}}_2}{{\text{H}}_6} + 2{\text{KOH}} + 2{{\text{H}}_2}{\text{O}}\xrightarrow{{}}2{\text{KB}}{{\text{O}}_2} + 6{{\text{H}}_2}({\text{xv}}){\text{ }}{{\text{B}}_2}{{\text{H}}_6} + 6{\text{C}}{{\text{l}}_2}\xrightarrow{{}}2{\text{BC}}{{\text{l}}_3} + 6{\text{HCl}}$
${{\text{B}}_2}{{\text{H}}_6} + 2{\text{LiH}}\xrightarrow{{}}2{\text{LiB}}{{\text{H}}_4}$
Aluminium
Extraction (Hall-Heroult Process):
Bauxite $\left( {{\text{A}}{{\text{l}}_2}{{\text{O}}_3} \cdot 2{{\text{H}}_2}{\text{O}}} \right)$ is the mineral ore from which aluminium is derived. Bayere's method is used to purify the ore initially. The anhydrous ${\text{A}}{{\text{l}}_2}{{\text{O}}_3}$ is combined with and fused. When aluminium is acquired at the cathode, the fused mixture of ${\text{N}}{{\text{a}}_{\text{3}}}{\text{Al}}{{\text{F}}_{\text{6}}}{\text{ \& Ca}}{{\text{F}}_{\text{2}}}$is treated to electrolytic reduction. Hoope's method purifies aluminium.
Properties:
Aluminium is a silvery metal with a density of$2.7\;{\text{g}}/{\text{cc}}$ and a melting point of $660^\circ {\text{C}}$ , as well as being an excellent heat and electrical conductor. It is ductile and malleable.
Action of Air: Dry air has no effect on aluminium. However, wet air creates a thin coating of ${\text{A}}{{\text{l}}_2}{{\text{O}}_3}$ on its surface, which dulls its brilliance. It burns to create ${\text{A}}{{\text{l}}_2}{{\text{O}}_3}$and ${\text{AlN}}$ at extremely high temperatures.
Reaction with Halogens:
When gaseous halogens travel through aluminum, the halide forms in an anhydrous state. $2{\text{Al}} + 3{\text{C}}{{\text{l}}_2}\xrightarrow{{}}2{\text{AlC}}{{\text{l}}_3}$
Action of Alkalies: When heated with concentrated ${\text{NaOH}}$, it releases ${{\text{H}}_2}$gas and forms a colorless sodium aluminate solution.
$2{\text{Al}} + 2{\text{NaOH}} + 2{{\text{H}}_2}{\text{O}}\xrightarrow{{}}2{\text{NaAl}}{{\text{O}}_2} + 3{{\text{H}}_2} \uparrow $
Action of Acids: Aluminium interacts with both dilute ${{\text{H}}_2}{\text{S}}{{\text{O}}_4}$and ${\text{HCl}}$but not with concentrated ${\text{HN}}{{\text{O}}_3}$, since concentrated ${\text{HN}}{{\text{O}}_3}$ causes aluminium to become passive, producing a protective oxide coating on the surface.
$2{\text{Al}} + 3{{\text{H}}_2}{\text{S}}{{\text{O}}_4}\xrightarrow{{}}{\text{A}}{{\text{l}}_2}{\left( {{\text{S}}{{\text{O}}_4}} \right)_3} + 3{{\text{H}}_2} \uparrow ;{\text{ }}2{\text{Al}} + 6{\text{HCl}}\xrightarrow{{}}2{\text{AlC}}{{\text{l}}_3} + 3{{\text{H}}_2} \uparrow $
Reaction with ${{\text{N}}_2}$ : Aluminum nitride is produced when ${{\text{N}}_2}$gas is passed over heated aluminum. As a result, hot aluminum functions as a ${{\text{N}}_2}$absorber.
$2{\text{Al}} + {{\text{N}}_2}\xrightarrow{{}}2{\text{AlN}}$
${\text{AlN}}$ reacts with hot water to form ${\text{Al}}{({\text{OH}})_3}$ and ${\text{N}}{{\text{H}}_3}$.
Reaction with Water: Cold water has no effect on aluminium. Boiling water or steam attacks it extremely slowly.
$2{\text{Al}} + 3{{\text{H}}_2}{\text{O}}\xrightarrow{{}}{\text{Al}}{({\text{OH}})_3} + 3{{\text{H}}_2} \uparrow $
Action of ${\text{HgC}}{{\text{l}}_2}$ Solution: Mercury is released when aluminium is added to a solution of ${\text{HgC}}{{\text{l}}_2}$.
$3{\text{HgC}}{{\text{l}}_2} + 2{\text{Al}}\xrightarrow{{}}2{\text{AlC}}{{\text{l}}_3} + 3{\text{Hg}} \downarrow $
Reduction of Oxides of Metals: When less reactive metal oxides are heated with aluminium; the less reactive metal is released. $3{\text{Mn}}{{\text{O}}_2} + 4{\text{Al}} + \xrightarrow{\Delta }2{\text{A}}{{\text{l}}_2}{{\text{O}}_3} + 3{\text{Mn}};{\text{ C}}{{\text{r}}_2}{{\text{O}}_3} + 2{\text{Al}} + \xrightarrow{\Delta }{\text{A}}{{\text{l}}_2}{{\text{O}}_3} + 2{\text{Cr}}$
Uses:
Aluminium is utilized for plating tanks, pipes, iron bars, and other steel items to prevent corrosion, manufacturing of aluminium cables, precise instruments, surgical apparatus, aircraft bodies, train coaches, motorboats, and automobiles.
Compounds of Aluminium:
Aluminium Oxide $\left( {{\text{A}}{{\text{l}}_2}{{\text{O}}_3}} \right)$ :
It's also known as alumina. It may be found in the form of bauxite and corundum in nature. It can also be found in gemstones. Topaz yellow, sapphire blue, ruby red, amethyst violet, and emerald green are some of the most important aluminium oxide stones.
Preparation:
Pure ${\text{A}}{{\text{l}}_2}{{\text{O}}_3}$ is obtained by igniting ${\text{A}}{{\text{l}}_2}{\left( {{\text{S}}{{\text{O}}_4}} \right)_3},{\text{Al}}{({\text{OH}})_3}$ or ammonium alum.
${\text{A}}{{\text{l}}_2}{\left( {{\text{S}}{{\text{O}}_4}} \right)_3} + \xrightarrow{\Delta }{\text{A}}{{\text{l}}_2}{{\text{O}}_3} + 3{\text{S}}{{\text{O}}_3} \uparrow ;{\text{ }}2{\text{Al}}{({\text{OH}})_3} + \xrightarrow{\Delta }{\text{A}}{{\text{l}}_2}{{\text{O}}_3} + 3{{\text{H}}_2}{\text{O}} \uparrow $
${\left( {{\text{N}}{{\text{H}}_4}} \right)_2}{\text{S}}{{\text{O}}_4} \cdot {\text{A}}{{\text{l}}_2}{\left( {{\text{S}}{{\text{O}}_4}} \right)_3} \cdot 24{{\text{H}}_2}{\text{O}}\xrightarrow{\Delta }2{\text{N}}{{\text{H}}_3} \uparrow + {\text{A}}{{\text{l}}_2}{{\text{O}}_3} + 4{\text{S}}{{\text{O}}_2} \uparrow + 25{{\text{H}}_2}{\text{O}} \uparrow $
Properties:
It's a white amorphous powder that's insoluble in water but soluble in acids (forming, for example${\text{AlC}}{{\text{l}}_3}$) and alkalies (forming${\text{NaAl}}{{\text{O}}_2}$), making it amphoteric. It's a polar covalent molecule with a positive charge.
Uses:
It is employed in the extraction of aluminium, the creation of fake gems, the production of aluminium compounds, and the fabrication of furnace linings. As a catalyst in organic processes, it is a refractory substance.
Aluminium Chloride $\left( {{\text{AlC}}{{\text{l}}_3},6{{\text{H}}_2}{\text{O}}} \right)$ :
It is a colourless crystalline solid that is water soluble. It has a covalent bond. Anhydrous ${\text{AlC}}{{\text{l}}_3}$is a white solid that is deliquescent.
Preparation:
By mixing aluminium, ${\text{A}}{{\text{l}}_2}{{\text{O}}_3}$, or ${\text{Al}}{({\text{OH}})_3}$ in dilute ${\text{HCl}}$ :
$2{\text{Al}} + 6{\text{HCl}}\xrightarrow{{}}2{\text{AlC}}{{\text{l}}_3} + 3{{\text{H}}_2} \uparrow ;{\text{ A}}{{\text{l}}_2}{{\text{O}}_3} + 6{\text{HCl}}\xrightarrow{{}}2{\text{AlC}}{{\text{l}}_3} + 3{{\text{H}}_2}{\text{O}};{\text{Al}}{({\text{OH}})_3} + 3{\text{HCl}}\xrightarrow{{}}{\text{AlC}}{{\text{l}}_3} + 3{{\text{H}}_2}{\text{O}}$
The solution gained is filtered and crystallized and the crystals of ${\text{AlC}}{{\text{l}}_3} \cdot 6{{\text{H}}_2}{\text{O}}$ are obtained.
Anhydrous ${\text{AlC}}{{\text{l}}_3}$ is obtained by the action of ${\text{C}}{{\text{l}}_2}$ on heated aluminium.
By heating a mixture of ${\text{A}}{{\text{l}}_2}{{\text{O}}_3}$ and coke and passing chlorine over it. ${\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{ + 3C + 3C}}{{\text{l}}_{\text{2}}}\xrightarrow{{}}{\text{2AlC}}{{\text{l}}_{\text{3}}}{\text{(anhy}}{\text{.) + 3CO}} \uparrow $
Properties:
Action of Heat:
When heated at a high temperature, hydrated salt is transformed to ${\text{A}}{{\text{l}}_2}{{\text{O}}_3}$. $2{\text{AlC}}{{\text{l}}_3} \cdot 6{{\text{H}}_2}{\text{O}}\xrightarrow{\Delta }{\text{A}}{{\text{l}}_2}{{\text{O}}_3} + 6{\text{HCl}} \uparrow + 9{{\text{H}}_2}{\text{O}}$
Action of Moisture on Anhydrous ${\text{AlC}}{{\text{l}}_3}$ :
When open to air, anhydrous ${\text{AlC}}{{\text{l}}_3}$ yields white fumes of ${\text{HCl}}$ ${\text{AlC}}{{\text{l}}_3} + 3{{\text{H}}_2}{\text{O}} \Leftrightarrow {\text{Al}}{({\text{OH}})_3} + 3{\text{HCl}} \uparrow $
Action of ${\text{N}}{{\text{H}}_3}$: Anhydrous ${\text{AlC}}{{\text{l}}_3}$ absorbs ${\text{N}}{{\text{H}}_3}$ since the latter is a Lewis acid. ${\text{AlC}}{{\text{l}}_3} + 6{\text{N}}{{\text{H}}_3} \to {\text{AlC}}{{\text{l}}_3} \cdot 6{\text{N}}{{\text{H}}_3}$ (white solid)
Action of ${\text{NaOH}}$ Solution:
When solution of ${\text{NaOH}}$is dropped into an aqueous solution of ${\text{AlC}}{{\text{l}}_3}$, a gelatinous precipitate of ${\text{Al}}{({\text{OH}})_3}$forms first, which dissolves in excess of ${\text{NaOH}}$solution to provide a colorless sodium aluminate solution.
${\text{AlC}}{{\text{l}}_3} + 3{\text{NaOH}} \to {\text{Al}}{({\text{OH}})_3} \downarrow + 3{\text{NaCl}};\quad {\text{Al}}{({\text{OH}})_3} + {\text{NaOH}} \to {\text{NaAl}}{{\text{O}}_2} + 2{{\text{H}}_2}{\text{O}}$
This reaction is useful for determining the difference between an aluminium salt and salts of${\text{Mg}},{\text{Ca}},{\text{Sr and Ba}}$. (When ${\text{NaOH}}$solution is added to their salt solutions, it creates a white hydroxide precipitate that does not dissolve in excess of${\text{NaOH}}$.)
Action of ${\text{N}}{{\text{H}}_4}{\text{OH}}$ Solution:
When ${\text{N}}{{\text{H}}_4}{\text{OH}}$ solution is combined with a solution of ${\text{AlC}}{{\text{l}}_3}$, a white precipitate of ${\text{Al}}{({\text{OH}})_3}$ is obtained which is insoluble in excess of ${\text{N}}{{\text{H}}_4}{\text{OH}}$.
${\text{AlC}}{{\text{l}}_3} + 3{\text{N}}{{\text{H}}_4}{\text{OH}} \to {\text{Al}}{({\text{OH}})_3} \downarrow $ (white gelatinous) $ + 3{\text{N}}{{\text{H}}_4}{\text{Cl}}$
To differentiate an ${\text{Al}}$salt from a ${\text{Zn}}$salt, this reaction is important. (When using a ${\text{Zn}}$salt, a white precipitate forms of ${\text{Zn}}{({\text{OH}})_2}$ that dissolves in excess of ${\text{N}}{{\text{H}}_4}{\text{OH}}$ solution.)
Hydrolysis with Water:
When ${\text{AlC}}{{\text{l}}_3}$is dissolved in water, it undergoes fast hydrolysis, yielding ${\text{Al}}{({\text{OH}})_3}$ which is a weak base and ${\text{HCl}}$ which is a strong acid. As a result, litmus finds the solution to be acidic.
${\left[ {{\text{Al}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{3 + }} \rightleftharpoons {\left[ {{\text{Al}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_5}{\text{OH}}} \right]^{ + 2}} + {{\text{H}}^ + }$
The complex cation has a great affinity to undergo dimerization.
$4{\text{LiH}} + {\text{AlC}}{{\text{l}}_3}\xrightarrow{{}}{\text{LiAl}}{{\text{H}}_4} + 3{\text{LiCl}}$
Uses:
It is employed as a catalyst in petroleum cracking, Friedel-Crafts processes, and the preparation of aluminium compounds.
Alums
${{\text{M}}_2}{\text{S}}{{\text{O}}_4} \cdot {\text{M}}_2^\prime {\left( {{\text{S}}{{\text{O}}_4}} \right)_3}.24{{\text{H}}_2}{\text{O}}$ OR ${\text{M}}{{\text{M}}^\prime }{\left( {{\text{S}}{{\text{O}}_4}} \right)_2}.12{{\text{H}}_2}{\text{O}}$
Alums are clear crystalline solids with the generic formula, where is a trivalent metal ${{\text{M}}^\prime }$and ${\text{M}}$ is a univalent metal or positive radical. Among the notable alums are:
Potash alum ${{\text{K}}_2}{\text{S}}{{\text{O}}_4} \cdot {\text{A}}{{\text{l}}_2}{\left( {{\text{S}}{{\text{O}}_4}} \right)_3} \cdot 24{{\text{H}}_2}{\text{O}}$, Chrome alum ${{\text{K}}_2}{\text{S}}{{\text{O}}_4} \cdot {\text{C}}{{\text{r}}_2}{\left( {{\text{S}}{{\text{O}}_4}} \right)_3} \cdot 24{{\text{H}}_2}{\text{O}}$, Ferric alum ${{\text{K}}_2}{\text{S}}{{\text{O}}_4} \cdot {\text{F}}{{\text{e}}_2}{\left( {{\text{S}}{{\text{O}}_4}} \right)_3} \cdot 24{{\text{H}}_2}{\text{O}}$, Ammonium alum ${\left( {{\text{N}}{{\text{H}}_4}} \right)_2}{\text{S}}{{\text{O}}_4} \cdot {\text{A}}{{\text{l}}_2}{\left( {{\text{S}}{{\text{O}}_4}} \right)_3} \cdot 24{{\text{H}}_2}{\text{O}}$
Alums are double salts that generate metal ions (or ammonium ions) and sulphate ions when dissolved in water.
Preparation:
Alums may be made by fusing ${{\text{M}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$and ${{\text{M'}}_2}{\left( {{\text{S}}{{\text{O}}_{\text{4}}}} \right)_{\text{3}}}$ in a 1: 1 molar ratio and dissolving the resultant material in water. Alums are crystallised from the resulting solution.
Uses:
It's used in the dye business as a mordant, as a germicide for water purification, and as a coagulating agent for removing colloidal contaminants from water.
(B) Group 14 Elements: The Carbon Family
Group 14 includes carbon (${\text{C}}$), silicon (${\text{Si}}$), germanium (${\text{Ge}}$), tin (${\text{Sn}}$), and lead (${\text{Pb}}$). There are two stable isotopes of carbon in nature: $^{ - 12}{\text{C}}$ and $^{13}{\text{C}}$. In addition to these third isotopes, $^{14}{\text{C}}$ is a radioactive isotope with a half-life of 5770 years that is utilised for radiocarbon dating. Ceramics, glass, and cement all contain significant amounts of silicon. Germanium is only found in trace amounts. Tin is typically found as cassiterite${\text{Sn}}{{\text{O}}_2}$, whereas lead is found as galena, ${\text{PbS}}$. Transistors and semiconductor devices are made from ultrapure forms of germanium and silicon.
Electronic Configuration:
The valence shell electronic configuration of these elements is ${\text{n}}{{\text{s}}^2}{\text{n}}{{\text{p}}^2}$.
Covalent Radius:
From ${\text{C to Si}}$, there is a significant rise in covalent radius, followed by a minor increase in radius from ${\text{Si to Pb}}$. The occurrence of completely filled ${\text{d and f}}$orbitals in heavier members explains this.
${\text{C}} < {\text{Si}} < {\text{Ge}} < {\text{Sn}} < {\text{Pb}}$${\text{C}} < {\text{Si}} < {\text{Ge}} < {\text{Sn}} < {\text{Pb}}$
Ionization Enthalpy:
The initial Ionization Enthalpy of members of group 14 is greater than that of members of group 13. The impact of the inner core electron may also be seen here. In general, the ionisation enthalpy ${\Delta _1}{\text{H}}$falls as one progresses through the group. The weak shielding effects of adjacent d and f orbitals, as well as increases in atom size, cause small decreases in from ${\text{Si to Ge to Sn}}$ and a tiny rise in $\Delta {\text{H}}$from Sn to ${\text{Pb}}$.
${\text{C}} < {\text{Si}} < {\text{Ge}} < {\text{Sn}} < {\text{Pb}}$${\text{C}} < {\text{Si}} < {\text{Ge}} < {\text{Sn}} < {\text{Pb}}$
Electronegativity:
The elements in this group are slightly more electronegative than those in group 13 due to their tiny size. From ${\text{Si to Pb}}$, the electronegativity values are nearly identical.
$\text{C}>\text{Si}\approx \text{Ge}\approx \text{Sn}\approx \text{Pb}$
Physical Properties:
All of the members of Group 14 are solids. Non-metals include carbon and silicon, metalloids include germanium, and soft metals like tin and lead have low melting points. The melting and boiling values of group 14 elements are significantly greater than those of group 13 elements.
Chemical Properties:
Oxidation States and Trends in Chemical Reactivity:
The outermost shell of group 14 elements has four electrons. These elements show $ + 4$ and $ + 2$ as the most frequent oxidation states and Carbon, which also has negative oxidation states. Compounds in the +4 oxidation state are usually covalent in nature because the sum of the first four ionisation enthalpies is relatively large. The tendency for heavier components to display$ + 2$ oxidation state rises as the sequence progresses ${\text{Ge}} < {\text{Sn}} < {\text{Pb}}$.
It is owing to the valence shell electrons ${\text{n}}{{\text{s}}^2}$inability to engage in bonding. The relative stability of these two oxidation states varies as one progresses through the group. Carbon can only surpass its covalence by a factor of four. Other members of the gang have the ability to do so. It's because they have the d orbital in them. Their halides are hydrolyzed as a result, and they have a proclivity for forming complexes by receiving electron pairs from donor species. For example, the species like, ${\text{SiF}}_6^{2 - } \cdot {\left[ {{\text{GeC}}{{\text{l}}_6}} \right]^{2 - }}$ ,${\left[ {{\text{Sn}}{{({\text{OH}})}_6}} \right]^{2 - }}$ exist.
Reactivity Towards Oxygen:
When heated in oxygen, all components produce oxides. There are primarily two forms of oxides: ${\text{MO}}$ and ${\text{M}}{{\text{O}}_2}$;monoxide and dioxide. Only at extremely high temperatures does ${\text{SiO}}$ exist. Higher oxidation state elements have more acidic oxides than lower oxidation state elements. The dioxides $ - {\text{C}}{{\text{O}}_2},{\text{Si}}{{\text{O}}_2}$ and ${\text{Ge}}{{\text{O}}_2}$ are acidic in nature, whereas ${\text{Sn}}{{\text{O}}_2}$ and ${\text{Pb}}{{\text{O}}_2}$ are amphoteric. Among monoxides, ${\text{CO}}$ is neutral, GeO is distinctly acidic whereas ${\text{SnO}}$ and ${\text{PbO}}$ are amphoteric.
Reactivity Towards Water:
Water has no effect on carbon, silicon, or germanium. Tin decomposes steam to produce dihydrogen gas and dioxide. Water has little effect on lead, owing to the development of a protective oxide coating.
Reactivity Towards Halogen:
These elements can create halides with the formula ${\text{M}}{{\text{X}}_2}$ and ${\text{M}}{{\text{X}}_4}$ (where $\left. {{\text{X}} = {\text{F}},{\text{ClBr}},{\text{l}}} \right).$all other components, except carbon, react directly with halogen under appropriate conditions to generate halides. The majority of them are ${\text{M}}{{\text{X}}_4}$ and are covalent. The only exceptions are ${\text{Sn}}{{\text{F}}_4}$ and ${\text{Pb}}{{\text{F}}_4}$,both of which are ionic in nature. ${\text{Pb}}{{\text{l}}_4}$ does not exist because ${\text{Pb - I}}$ bond initially formed during the reaction does not release enough energy to unpair $6\;{{\text{s}}^2}$ electrons and excite one of them to higher orbital to have four unpaired electrons around lead atom. Heavier members Ge to ${\text{Pb}}$ are able to make halides of formula ${\text{M}}{{\text{X}}_2}$. Stability of dihalides increases down the group. Water may easily hydrolyze other tetrachlorides but not ${\text{CC}}{{\text{l}}_4}$because the central atoms except carbon can accept the lone pair of electrons from the oxygen atom of water molecules in the d orbital.
Important Trends And Anomalous Behaviour Of Carbon
Carbon, like the initial member of other groupings, is distinct from the rest of its peers. Its smaller size, greater electronegativity, higher ionisation enthalpy, and lack d- orbitals, all contribute to this anomalous behaviour of carbon. Only four pairs of electrons can be accommodated around it. Carbon has the unique ability to form $p\pi - p\pi $ multiple bonds with itself and other atoms of small size and high electronegativity, whereas other members can expand their covalence due to the presence of d orbitals. Carbon also has the unique ability to form $p\pi - p\pi $ multiple bonds with itself and other atoms of small size and high electronegativity. Few example of multiple bonding are ${\text{C}} = {\text{C}},{\text{C}} \equiv {\text{C}},{\text{C}} = {\text{O,C}} = {\text{S}}$ and ${\text{C}} \equiv {\text{N}}$.Because the atomic orbitals of heavier elements are too vast and diffuse to have effective overlapping, they do not ${\text{p\pi - p\pi }}$ form bonds. Carbon atoms have a natural inclination to build chains and rings by forming covalent connections with one another. Catenation is the term for this feature. Because ${\text{C - C}}$ bonds are extremely strong, this is the case. As the size of the group grows larger, the likelihood to display catenation reduces. Bond enthalpies values clearly demonstrate this. Catenation is done in the order ${\text{C}} > > {\text{Si}} > {\text{Ge}} \approx {\text{Sn}}$. Catenation does not occur in lead. Carbon may take on allotropic forms due to its catenation and $ p\pi - p\pi $ bond forming properties.
Allotropes of Carbon
Carbon exists in a variety of allotropic forms, both crystallic and amorphous. Two well-known crystalline forms of carbon are diamond and graphite. H.W. Kroto, E. Smalley, and R.F. Curl discovered fullerenes, a third form of carbon, in 1985.
Diamond:
Its lattice is crystalline. Each carbon atom in a diamond undergoes sp hybridisation and is connected to four other carbon atoms in a tetrahedral manner utilising hybridised orbitals. The length of the C-C bond is $154{\text{pm}}$. The structure expands into space, forming a three-dimensional network of carbon atoms that is hard. Directional covalent bonds are found across the lattice in this structure. Diamond is the hardest substance on the planet because it is extremely difficult to break prolonged covalent bonds. It's used as an abrasive for sharpening hard instruments, and it's also used to make dyes and tungsten filament for light bulbs.
Graphite:
The structure of graphite is layered. Van der Waal's forces hold the layers together, and the distance between them is 340 pm. Each layer is made up of carbon atoms arranged in planar hexagonal rings. Within the layer, the C - C bond length is 141.5 pm. Each carbon atom in a hexagonal ring goes through sp2 hybridisation and forms three sigma bonds with three carbon atoms nearby. A ${\pi}$ bond is formed by the fourth electron. The electrons are dispersed throughout the sheet. Because electrons are mobile, graphite transmits electricity across the sheet. Graphite is particularly soft and slippery because it cleaves easily between the layers. As a result, graphite is utilised as a dry lubricant in machines that operate at high temperatures and cannot use oil.
Fullerenes:
Fullerenes are created by heating graphite in the presence of inert gases such as helium or argon in an electrical arc. Because of its smooth shape and lack of 'dangling' connections, fullerenes are the only pure form of carbon. Fullerenes are a kind of molecule that resembles a cage. Buckminsterfullerene is a ${{\text{C}}_{{\text{60}}}}$ molecule with the form of a soccer ball. There are twenty 6-membered rings and twelve five-membered rings in this set. A six-membered ring can be fused with other six-membered rings, while a five-membered ring can only be fused with other six-membered rings. The carbon atoms are all the same and undergo ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridization. Three sigma bonds occur between each carbon atom and the other three carbon atoms. Each carbon atom's remaining electron is delocalized in molecular orbitals, giving the molecule its aromatic nature. This molecule is structured like a ball and has 60 vertices, each of which is occupied by one carbon atom. It also has single and double bonds with distances of${\text{C - C}}$ are $143.5{\text{pm}}$ and $138.3{\text{pm}}$ respectively. Bucky balls are the short name for spherical fullerenes. It's crucial to remember that graphite is the most thermodynamically stable allotrope of carbon, therefore diamond and fullerene have${\Delta _1}{{\text{H}}^{( - )}}$ values of 1.90 and$38.1\;{\text{kJ}}\;{\text{mo}}{{\text{l}}^{ - 1}}$ respectively. Carbon black is made by burning hydrocarbons with a little amount of oxygen.
Uses of Carbon:
High-strength, lightweight composites are made from graphite fibres embedded in plastic. Tennis rackets, fishing rods, aeroplanes, and canoes are all made from composites. Graphite is used for electrodes in batteries and industrial electrolysis because it is an excellent conductor. Graphite crucibles are inert to dilute acids and alkalies. Activated charcoal is used to absorb harmful gases, as well as in water filters to remove organic contaminants and air conditioning systems to regulate smell. Carbon black is used as a filler in car tyres and as a dark pigment in black ink. Coke is primarily utilised as a reducing agent in metallurgy and as a fuel. Diamond is a valuable stone that is used in jewellery.
Properties Of Carbon:
Carbon in any form will react with oxygen at a sufficiently high temperature to give carbon dioxide; in a deficiency of oxygen, carbon monoxide is formed as well.
${\text{C}}({\text{s}}) + 2\;{\text{S}}(\;{\text{s}})\xrightarrow{{}}{\text{C}}{{\text{S}}_2}(1)$
${\text{Ca}}({\text{s}}) + 2{\text{C}}({\text{s}})\xrightarrow{{}}{\text{Ca}}{{\text{C}}_2}(\;{\text{s}})$
${\text{C}}({\text{s}}) + 2\;{{\text{F}}_2}(\;{\text{g}})\xrightarrow{{}}{\text{C}}{{\text{F}}_4}(\;{\text{g}})$
It will decrease steam, creating water gas and a variety of metal oxides; these reductions are important for industry.
${\text{C}} + {{\text{H}}_2}{\text{O}}({\text{g}})\xrightarrow{\Delta }{\text{CO}} + {{\text{H}}_2};{\text{ F}}{{\text{e}}_2}{{\text{O}}_3} + 3{\text{C}}\xrightarrow{{}}2{\text{Fe}} + 3{\text{CO}}$
It is not attacked by dilute acids, but concentrated nitric acid and sulphuric acid are reduced if warmed with carbon according to the equations:
${\text{C}}({\text{s}}) + 4{\text{HN}}{{\text{O}}_3}({\text{aq}})\xrightarrow{{}}2{{\text{H}}_2}{\text{O}}(1) + 4{\text{N}}{{\text{O}}_2}(\;{\text{g}}) + {\text{C}}{{\text{O}}_2}(\;{\text{g}});{\text{ C}}({\text{s}}) + 2{{\text{H}}_2}{\text{S}}{{\text{O}}_4}(1)\xrightarrow{{}}2{{\text{H}}_2}{\text{O}}(1) + 2{\text{S}}{{\text{O}}_2}(\;{\text{g}}) + {\text{C}}{{\text{O}}_2}(\;{\text{g}})$
Oxides Of Carbon:
Carbon Dioxide :$CO_{2}$
Preparation:
It is easily produced in the laboratory by the action of weak hydrochloric acid on marble chips:
${\text{CO}}_3^{2 - }({\text{aq}}) + 2{{\text{H}}^ + }({\text{aq}})\xrightarrow{{}}{\text{C}}{{\text{O}}_2}(\;{\text{g}}) + {{\text{H}}_2}{\text{O}}(1)$
It is generated as a by-product in the manufacturing of quicklime and in fermentation operations in the industrial sector: ${\text{CaC}}{{\text{O}}_3}(\;{\text{s}})\xrightarrow{{}}{\text{CaO}}({\text{s}}) + {\text{C}}{{\text{O}}_2}(\;{\text{g}});{\text{ }}{{\text{C}}_6}{{\text{H}}_{12}}{{\text{O}}_6}({\text{aq}})\{ {\text{ glucose }}\} \to 2{{\text{C}}_2}{{\text{H}}_5}{\text{OH}}({\text{aq}}) + 2{\text{C}}{{\text{O}}_2}(\;{\text{g}})$
Properties:
At normal temperature and pressure, it is a colourless, odourless, and heavy gas that dissolves in its own volume of water. When the pressure is increased, it dissolves considerably more quickly in water, as do all gases, and this concept is employed in the production of soda water and fizzy beverages.
${\text{C}}{{\text{O}}_2}$is quickly liquefied (critical temperature $ = 31.1^\circ {\text{ C}}$ ), and a cylinder of the gas under pressure serves as a handy fire extinguisher. Solid carbon dioxide ('dryice') is created when a highly compressed gas is allowed to expand fast. Because no massy liquid is generated when solid carbon dioxide sublimes at $ - {78^\circ }{\text{C}}$, it is a handy way to produce low temperatures.
Carbon dioxide is the anhydride of carbonic acid, a weak dibasic acid that ionises in the following order:
${{{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}{\text{(aq) +}}{{\text{H}}_{\text{2}}}{\text{O(1) ( reversible ) HCO}}_{\text{3}}^{\text{ - }}{\text{(aq) + }}{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}{\text{(aq)}}} $
${{\text{HCO}}_{\text{3}}^{\text{ - }}{\text{(aq) + }}{{\text{H}}_{\text{2}}}{\text{O(1) (reversible) CO}}_{\text{3}}^{{\text{2 - }}}{\text{(aq) + }}{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}{\text{(aq) }}} $
The buffer system of
${{\text{H}}_2}{\text{C}}{{\text{O}}_3}/{\text{HCO}}_3^ - $ aids in maintaining blood ${\text{pH}}$ in the range of 7.26 to 7.42. When a solution of carbonic acid in water is progressively turned blue litmus red, all of ${\text{C}}{{\text{O}}_2}$ is released when the solution is boiled.
Carbon dioxide readily reacts with alkalis forming the carbonate and, if ${\text{C}}{{\text{O}}_2}$ is in excess, the hydrogen carbonate. This is the basis of the lime-water test for ${\text{C}}{{\text{O}}_2}$ gas.
When carbon dioxide interacts with alkalies, it forms carbonate and ${\text{C}}{{\text{O}}_2}$, if there is a surplus, hydrogen carbonate. The lime-water test for${\text{C}}{{\text{O}}_2}$ gas is based on this.
${{\text{Ca}}{{({\text{OH}})}_2}({\text{aq}})+{\text{C}}{{\text{O}}_2}(\;{\text{g}}) \to {\text{CaC}}{{\text{O}}_3}(\;{\text{s}}) + {{\text{H}}_2}{\text{O}}({\text{liq}})} $
${{\text{CaC}}{{\text{O}}_3}(\;{\text{s}})+{{\text{H}}_2}{\text{O}}({\text{liq}})+{\text{C}}{{\text{O}}_2}(\;{\text{g}})\to{\text{Ca}}{{\left( {{\text{HC}}{{\text{O}}_3}} \right)}_2}({\text{aq}})} $
The above reaction accounts for the formation of temporarily hard water.
Carbon dioxide, which is normally present to the extent of 0.03% by volume in the atmosphere, is removed from it by the process known as photosynthesis. It is the process by which green plants convert atmospheric ${\text{C}}{{\text{O}}_2}$ into carbohydrates such as glucose. The overall chemical change can be expressed as:
The process of photosynthesis removes carbon dioxide from the atmosphere, which is typically present to the level of 0.03% by volume. It is the process by which green plants transform ${\text{C}}{{\text{O}}_2}$ from the atmosphere into carbohydrates like glucose. The total chemical shift can be represented as:
${\text{6C}}{{\text{O}}_{\text{2}}}{\text{ + 12}}{{\text{H}}_{\text{2}}}{\text{O}}\xrightarrow[{{\text{chlorophyll}}}]{{{\text{h\nu }}}}{{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}{\text{ + 6}}{{\text{O}}_{\text{2}}}{\text{ + 6}}{{\text{H}}_{\text{2}}}{\text{O}}$
Plants use this process to produce food for themselves, as well as animals and humans. However, recent increases in fossil fuel burning and limestone breakdown for cement manufacturing appear to have increased ${\text{C}}{{\text{O}}_2}$ levels in the atmosphere. This might lead to an increase in the greenhouse effect and, as a result, a rise in atmospheric temperature, which could have significant implications.
Carbonation of soft drinks is commonly done using gaseous ${\text{C}}{{\text{O}}_2}$. It is used as a fire extinguisher since it is heavy and does not encourage combustion. The production of urea consumes a significant amount of${\text{C}}{{\text{O}}_2}$.
Carbon Monoxide (Co):
Preparation:
When carbon or carbonaceous materials is oxidised by air or oxygen, it produces carbon monoxide with ${\text{C}}{{\text{O}}_2}$. It is also generated when ${\text{C}}{{\text{O}}_2}$is reduced using red-hot carbon; this reaction is important in metal extractions.
${\text{C}}({\text{s}}) + {\text{C}}{{\text{O}}_2}(\;{\text{g}})\xrightarrow{{}}2{\text{CO}}({\text{g}})$
It may be made in the lab by dehydrating methanoic acid and mixing it with concentrated sulphuric acid:
\[{\text{HCOOH(liq)}}\xrightarrow[{{\text{conc}}{\text{.}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}]{{{\text{373K}}}}{\text{CO(g) + }}{{\text{H}}_{\text{2}}}{\text{O}}\]
When oxalic acid is dehydrated in the same way, oxalic acid is also produced.${{\text{H}}_2}{{\text{C}}_2}{{\text{O}}_4}\dfrac{{{\text{ conc}}{\text{. }}{{\text{H}}_2}{\text{S}}{{\text{O}}_4},\Delta }}{{ - {{\text{H}}_2}{\text{O}}}}{\text{CO}} + {\text{C}}{{\text{O}}_2}$
Steam is passed over heated coke on a commercial scale to prepare it. Water gas, also known as synthesis gases, is the combination of ${\text{CO}}$ and ${{\text{H}}_2}$.
${\text{C(s) + }}{{\text{H}}_{\text{2}}}{\text{O(g)}}\xrightarrow[{{\text{473 - 1273K}}}]{}{\text{CO(g) + }}{{\text{H}}_{\text{2}}}\;{\text{(g)}}$ (water gas)
When air is utilized instead of steam, it produces a combination of ${\text{CO}}$ and ${{\text{N}}_2}$ known as producer gas.
${\text{2C(s) + }}{{\text{O}}_{\text{2}}}{\text{(g) + 4}}{{\text{N}}_{\text{2}}}{\text{(g)}}\xrightarrow{{{\text{1273K}}}}{\text{2CO(g) + 4}}{{\text{N}}_{\text{2}}}{\text{(g) (producer gas)}}$
Industrial fuels such as water gas and producer gas are highly essential. With the release of heat, carbon monoxide in water gas or producer gas can be further combusted to generate carbon dioxide.
${\text{Zn}}+{\text{C}}{{\text{O}}_2}\xrightarrow{{}}{\text{ZnO}} + {\text{CO}}$
$k_{4}Fe(CN)_{6}+6H_2S0_{4}(Conc.)+6H_2O \overset{\Delta }{\rightarrow}2K_{2}SO_{4}+FeSO_4+3(NH_4)_2SO_4+6CO$
${\text{HCN}}+2{{\text{H}}_2}{\text{O}}\xrightarrow{{}}{\text{HCOOH}} + 2{\text{N}}{{\text{H}}_3}$ (absorbed by$\left. {{{\text{H}}_2}{\text{S}}{{\text{O}}_4}} \right)$
${\text{HCOOH}}\xrightarrow{\Delta }{{\text{H}}_2}{\text{O}} + {\text{CO}}$
Properties:
Carbon monoxide is a colourless, odourless gas that forms a blue flame when burned in air. It is very toxic, interacting with haemoglobin in the blood more readily than oxygen, causing rapid obstructing of normal breathing. Because the gas is not quickly absorbed by active charcoal, ordinary gas masks provide no protection. because it does not readily bind to active charcoal A combination of manganese (IV) oxide and copper (II) oxide catalytically oxidises it to ${\text{C}}{{\text{O}}_2}$ in the presence of air, and this combined catalyst is employed in the breathing equipment worn by rescue crews during mining catastrophes.
Carbon monoxide is a strong reducing agent that is used in the extraction of iron and nickel in industry:
${\text{F}}{{\text{e}}_2}{{\text{O}}_3}(\;{\text{s}}) + 3{\text{CO}}({\text{g}})\xrightarrow{{}}2{\text{Fe}}({\text{s}}) + 2{\text{C}}{{\text{O}}_2}(\;{\text{g}});{\text{ NiO}}({\text{s}}) + {\text{CO}}({\text{g}})\xrightarrow{{}}{\text{Ni}}({\text{s}}) + {\text{C}}{{\text{O}}_2}(\;{\text{g}})$
It interacts with a variety of transition metals to produce volatile carbonyls; the production of nickel carbonyl followed by its breakdown is the foundation of Mond's technique for getting extremely pure nickel:
${\text{Ni(s) + 4CO(g)}}\xrightarrow{{{\text{9}}{{\text{0}}^{\text{o}}}{\text{C}}}}{\text{ Ni(CO}}{{\text{)}}_{\text{4}}}{\text{(liq) }}\xrightarrow{{{\text{18}}{{\text{0}}^{\text{o}}}{\text{C}}}}{\text{Ni(s) + 4CO(g)}}$
Carbon monoxide reacts with sulphur to form carbonyl sulphide, and with chlorine in the presence of light to form carbonyl chloride (phosgene), which is used in the manufacture of polyurethane foam plastics. Phosgene is an extremely toxic gas.
${\text{CO}}({\text{g}})+{\text{S}}({\text{s}})\xrightarrow{{}}{\text{ COS}}({\text{s}}) $
$ {\text{carbonyl sulphide }}$
${\text{CO}}({\text{g}})+{\text{C}}{{\text{l}}_2}(\;{\text{g}})\xrightarrow{{}}{\text{COC}}{{\text{l}}_2}(\;{\text{g}}) $
$ {\text{ carbonyl chloride}}$
Although carbon monoxide is not a genuine acid anhydride since it does not generate an acid when it interacts with water, it does produce sodium methanoate when it reacts under pressure with fused sodium hydroxide:
${\text{NaOH(liq) + CO(g)}}\xrightarrow{{}}{\text{ HCOONa(s) }}\xrightarrow{{{\text{dil}}{\text{.HCl}}}}{\text{HCOOH(aq)}}$
It combines with hydrogen under pressure in the presence of a zinc oxide or chromium (III) oxide catalyst to produce methanol; this is an important industrial process. ${\text{CO}}({\text{g}}) + 2{{\text{H}}_2}(\;{\text{g}})\xrightarrow{{}}{\text{C}}{{\text{H}}_3}{\text{OH}}({\text{liq}})$
${\text{CO}}$ is readily absorbed by an ammoniacal solution of copper (I) chloride to give ${\text{CuClCO}}.2{{\text{H}}_2}{\text{O}}$. It reduces an ammonical solution of silver nitrate to silver (black) and, in the absence of other gaseous reducing agents, this serves as a test for the gas. It can be estimated byreaction with iodine pentoxide, the iodine which is produced quantitatively being titrated with standard sodium thiosulphate solution.
An ammoniacal solution of copper (I) chloride easily absorbs by${\text{CO}}$ to yield ${\text{CuClCO}}.2{{\text{H}}_2}{\text{O}}$. it reduces an ammonical solution of silver nitrate to silver (black), and this acts as a test for the gas. It may be calculated using an iodine pentoxide reaction, with the iodine generated being titrated using a standard sodium thiosulphate solution.
$5{\text{CO}}({\text{g}}) + {{\text{I}}_2}{{\text{O}}_5}(\;{\text{s}})\xrightarrow{{}}{{\text{I}}_2}(\;{\text{s}}) + 5{\text{C}}{{\text{O}}_2}(\;{\text{g}})$
Carbon Suboxide $C_{3}O_{2}$
The anhydride of propanedioic acid (malonic acid), of which it is the anhydride, may be produced by dehydrating it with phosphorus pentoxide: $3{\text{C}}{{\text{H}}_2}{({\text{COOH}})_2} + {{\text{P}}_4}{{\text{O}}_{10}}\xrightarrow{{}}3{{\text{C}}_3}{{\text{O}}_2} + 4{{\text{H}}_3}{\text{P}}{{\text{O}}_4}$
When heated to about $ = {200^\circ }{\text{ C}}$ , it decomposes into ${\text{C}}{{\text{O}}_2}$ and ${\text{C}}$ :
${{\text{C}}_3}{{\text{O}}_2}(\;{\text{g}})\xrightarrow{{}}{\text{C}}{{\text{O}}_2}(\;{\text{g}}) + 2{\text{C}}({\text{s}})$
The molecule is thought to have a linear structure: ${\text{O}} = {\text{C}} = {\text{C}} = {\text{C}} = {\text{O}}$.
Carbonates $CO_{3}^{2-}$ And Bicarbonates$(HC0_{3}^{-})$
Carbonic acid is a dibasic acid that produces two types of salts: carbonates (normal salts) and bicarbonates (acid salts) when the replacement hydrogens from ${{\text{H}}_2}{\text{C}}{{\text{O}}_3}$.
${{\text{H}}_2}{\text{C}}{{\text{O}}_3} + {\text{NaOH}}\xrightarrow{{}}{\text{NaHC}}{{\text{O}}_3} + {{\text{H}}_2}{\text{O}};{\text{ NaHC}}{{\text{O}}_3} + {\text{NaOH}}\xrightarrow{{}}{\text{N}}{{\text{a}}_2}{\text{C}}{{\text{O}}_3} + {{\text{H}}_2}{\text{O}}$
Preparation:
With ${\text{NaOH}}$ :
$2{\text{NaOH}} + {\text{C}}{{\text{O}}_2}\xrightarrow{{}}{\text{N}}{{\text{a}}_2}{\text{C}}{{\text{O}}_3};{\text{ N}}{{\text{a}}_2}{\text{C}}{{\text{O}}_3} + {{\text{H}}_2}{\text{O}} + {\text{C}}{{\text{O}}_2}\xrightarrow{{}}2{\text{NaHC}}{{\text{O}}_3}$
By Precipitation:
${\text{BaC}}{{\text{l}}_2} + {\text{N}}{{\text{a}}_2}{\text{C}}{{\text{O}}_3}\xrightarrow{{}}{\text{BaC}}{{\text{O}}_3} \downarrow + 2{\text{NaCl}}$
Carbides:
Carbides are carbon-based binary compounds with additional elements that are less electronegative or have equivalent electronegativity. They are divided into three groups: ionic, covalent, and interstitial (or metallic)
Ionic Carbides (or Salt like Carbides): Usually made up of components from the I, II, and III groups (Boron is an exception). They are further sub-classified into three categories based on the hydrolysis product.
Methanides These give ${\text{C}}{{\text{H}}_4}$ on reaction with ${{\text{H}}_2}{\text{O}}$. ${\text{A}}{{\text{l}}_4}{{\text{C}}_3} + 12{{\text{H}}_2}{\text{O}}\xrightarrow{{}}4{\text{A}}{{\text{l}}_{\text{2}}}{({\text{OH}})_3} + 3{\text{C}}{{\text{H}}_4};{\text{ B}}{{\text{e}}_2}{\text{C}} + 4{{\text{H}}_2}{\text{O}}\xrightarrow{{}}2{\text{Be}}{({\text{OH}})_2} + {\text{C}}{{\text{H}}_4}$
These carbides contain ${{\text{C}}^{4 + }}$ ions in their constitution.
Acetylides: These give ${{\text{C}}_2}{{\text{H}}_2}$ on reaction with ${{\text{H}}_2}{\text{O}}$.
${{\text{Ca}}{{\text{C}}_2}+2{{\text{H}}_2}{\text{O}}\xrightarrow{{}}{\text{Ca}}{{({\text{OH}})}_2}+{{\text{C}}_2}{{\text{H}}_2};{\text{ A}}{{\text{l}}_2}{{\left( {{{\text{C}}_2}} \right)}_3} + 6{{\text{H}}_2}{\text{O}}\xrightarrow{{}}2{\text{Al}}{{({\text{OH}})}_3} + 3{{\text{C}}_2}{{\text{H}}_2}} $
${{\text{Sr}}{{\text{C}}_2}+2{{\text{H}}_2}{\text{O}}\xrightarrow{{}}{\text{Sr}}{{({\text{OH}})}_2} + {{\text{C}}_2}{{\text{H}}_2}} $
Such compounds contain ${\text{C}}_{\text{2}}^{{\text{2 - }}}{{\text{[:C}} \equiv {\text{C:]}}^{{\text{2 - }}}}{\text{ions}}$.
Allylides These give 1-propyne on reaction with ${{\text{H}}_2}{\text{O}}$.
${\text{M}}{{\text{g}}_2}{{\text{C}}_3} + 4{{\text{H}}_2}{\text{O}}\xrightarrow{{}}2{\text{Mg}}{({\text{OH}})_2} + {\text{C}}{{\text{H}}_3} - {\text{C}} \equiv {\text{CH}}$
Such compounds contain ${{\text{C}}_3}^4{[:\mathop {\text{C}}\limits^{..} - {\text{C}} \equiv {\text{C}}:]^{4 - }}$ ions
Covalent Carbides Compounds (like ${\text{C}}{{\text{H}}_4},{\text{C}}{{\text{O}}_2},{\text{C}}{{\text{S}}_2}$ ) these are some giant molecules like $\operatorname{SiC} $ are also examples of covalent carbides.
Interstitial or metallic carbides: Transition metals, in which carbon atoms occupy interstitials in the crystal structure of metals, create such carbides.
Carborundum $Sic$.
Preparation:
${\text{Si}}{{\text{O}}_{\text{2}}}{\text{ + 3C}}\xrightarrow[{{\text{furnance200}}{{\text{0}}^{\text{o}}}{\text{C}}}]{{{\text{elec}}{\text{.}}}}\mathop {{\text{ SiC}}}\limits_{} {\text{ + 2CO}}$
Properties:
It's a brittle material(Hardness$ = 9.5{\text{Moh}}$). It does not melt when heated, but rather decomposes into components. Acids have no effect on it. At high temperatures, however, it produces the following two reactions. Each atom is ${\text{s}}{{\text{p}}^3}$ hybridised, giving it a diamond-like structure. As a result, each atom is surrounded by four atoms of the opposite kind.
${\text{SiC}} + 2{\text{NaOH}} + 2{{\text{O}}_2}\xrightarrow{\Delta }{\text{N}}{{\text{a}}_2}{\text{Si}}{{\text{O}}_3} + {\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}};{\text{ SiC}} + 4{\text{C}}{{\text{l}}_2}\xrightarrow{\Delta }{\text{SiC}}{{\text{l}}_4} + {\text{CC}}{{\text{l}}_4}$
Silicon:
Silicon is the second most prevalent element in the earth's crust (approximately 28% by weight) and may be found as the oxide silica in a number of forms, such as sand, quartz, and flint, as well as silicates in rocks and clays.
Preparation:
By reducing silica with carbon in an electric furnace, the element can be obtained: ${\text{Si}}{{\text{O}}_2}(\;{\text{s}}) + 2{\text{C}}({\text{s}})\xrightarrow{{}}{\text{Si}}({\text{s}}) + 2{\text{CO}}({\text{g}})$
The process of zone refining is used to generate very pure silicon from 'chemically' pure silicon. (2) ${\text{Si}}{{\text{O}}_2} + 2{\text{Mg}}\xrightarrow{\Delta }2{\text{MgO}} + {\text{Si}}$
Properties:
Silicon is a high-melting-point solid with a diamond-like structure. The lack of an allotrope with the graphite structure demonstrates silicon atoms' inability to multiple bind with one another. Silicon is chemically inert in its bulk state, but halogens and alkalies attack it when it is powdered:
${\text{Si(powdered) + 2C}}{{\text{l}}_{\text{2}}}{\text{(g)}}\xrightarrow{{}}{\text{ SiC}}{{\text{l}}_{\text{4}}}{\text{(liq)}}$
${\text{Si}}\left( {{\text{powdered}}} \right){\text{ + 2O}}{{\text{H}}^{{\text{ - }}}}{\text{(aq) + }}{{\text{H}}_{\text{2}}}{\text{O (liq) }}\xrightarrow{{}}{\text{SiO}}_{\text{3}}^{{\text{2 - }}}{\text{(aq) + 2}}{{\text{H}}_{\text{2}}}(\;{\text{g}})$
Except for hydrofluoric acid, with which it produces hexafluorosilicic acid, it is unaffected by acids.
${\text{Si(s) + 6HF(g)}}\xrightarrow{{{{\text{H}}_{\text{2}}}}}{\text{ Si}}{{\text{F}}_{\text{6}}}{\text{(aq) + 2}}{{\text{H}}_{\text{2}}}(\;{\text{g}})$
${\text{Si}} + 2{\text{KOH}} + {{\text{H}}_2}{\text{O}}\xrightarrow{{}}{{\text{K}}_2}{\text{Si}}{{\text{O}}_3} + 2{{\text{H}}_2}$
${\text{N}}{{\text{a}}_2}{\text{C}}{{\text{O}}_3} + {\text{Si}}\xrightarrow{\Delta }{\text{N}}{{\text{a}}_2}{\text{Si}}{{\text{O}}_3} + {\text{C}}$
$2{\text{Mg}} + {\text{Si}}\xrightarrow{{}}{\text{M}}{{\text{g}}_2}{\text{Si}}$ (Magnesium silicide)
Compounds of Silicon:
Silicon dioxide, ${\text{Si}}{{\text{O}}_2}$
Silicon dioxide, ${\text{Si}}{{\text{O}}_2}$ or silica, comes in a variety of crystallographic shapes. Some crystalline forms of silica, such as quartz, cristobalite, and tridymite, can be interconverted at the right temperature. Silicon dioxide is a three-dimensional covalent network solid in which each silicon atom is covalently linked to four oxygen atoms in a tetrahedral arrangement. Each oxygen atom then formed a covalent connection with a silicon atom. Each tetrahedron shares a corner with another tetrahedron. The whole crystal may be thought of as a large molecule with eight membered rings made by silicon and oxygen atoms alternated. Because of its high${\text{Si}} - {\text{O}}$ bond enthalpy, silica is practically nonreactive in its natural state. Even at high temperatures, it is resistant to halogens, dihydrogen, and most acids and metals. HF and ${\text{NaOH}}$, on the other hand, attack it.
${{\text{Si}}{{\text{O}}_2}+2{\text{NaOH}}\xrightarrow{{}}{\text{N}}{{\text{a}}_2}{\text{Si}}{{\text{O}}_3}+{{\text{H}}_2}{\text{O}}} $
${{\text{Si}}{{\text{O}}_2}+4{\text{HF}}\xrightarrow{{}}{\text{Si}}{{\text{F}}_4} + 2{{\text{H}}_2}{\text{O}}} $
Quartz is widely utilised as a piezoelectric material, allowing for the development of ultra-reliable clocks, contemporary radio and television transmission, and mobile radio communications. Silica gel is a drying agent that may also be used to support chromatographic materials and catalysts. In filtration facilities, Kieselghur, an amorphous type of silica, is utilised.
Silicates:
Silicates are binary silicon-oxygen compounds that also contain additional metals in their atomic structures.
Because the difference in negativity between${\text{O}}$and ${\text{Si}}$ is roughly 1.7, the link can be classified as $50\% $ ionic & $50\% $ covalent.
If we calculate the radius ratio $\dfrac{{{r_{S{i^{ - 4}}}}}}{{{r_{{0^2} - }}}} = 0.29$ It suggests that the co-ordination no. of silicon must be 4 and from VBT point of view we can say that Si is sp $^3$ hybridized. Therefore silicate structures must be based upon ${\text{SiO}}_4^{ - 4}$ tetrahedral units
${\text{SiO}}_4^{ - 4}$ tetrahedral units may exist as discrete units or may polymerise into larger units by sharing corners.
Classification Of Silicates:
Orthosilicates:
As seen in the picture, these include distinct units of ${\left[ {{\text{Si}}{{\text{O}}_4}} \right]^4}$, i.e. there is no sharing of corners.
Pyrosilicate:
Two tetrahedral units are linked in these silicates by sharing oxygen at one comer, resulting in ${\left[ {{\text{S}}{{\text{i}}_2}{{\text{O}}_7}} \right]^{6 - }}$units.
The oxygen atoms that are coupled with one Siatom will have a negative charge. Thorteveitite $\left( {{\text{S}}{{\text{c}}_2}{\text{S}}{{\text{i}}_2}{{\text{O}}_7}} \right)$and Hemimorphite, for example. $\left( {{\text{Z}}{{\text{n}}_3}\left( {{\text{S}}{{\text{i}}_2}{{\text{O}}_7}} \right){\text{Zn}}{{({\text{OH}})}_2}{{\text{H}}_2}{\text{O}}} \right)$
Cyclic Silicates:
The silicates containing these anions are known as cyclic silicates because two oxygen atoms per tetrahedron are shared to form closed rings, resulting in the structure with general formula ${\left( {{\text{SiO}}_3^{2 - }} \right)_{\text{n}}}$ or $\left( {{\text{Si}}{{\text{O}}_3}} \right)_{\text{n}}^{22}$ Typical examples of cyclic silicates include ${\text{S}}{{\text{i}}_3}{\text{O}}_9^{6 - }$ and ${\text{S}}{{\text{i}}_6}{\text{O}}_{18}^{12 - }$ anions.
Chain Silicates:
Simple chain and double chain chain silicates are two types of chain silicates. In simple chains, two corners of each tetrahedron are shared, resulting in a lengthy tetrahedron chain. They have the same general formula as cyclic silicates, i.e. $\left( {{\text{Si}}{{\text{O}}_3}} \right)_{\text{n}}^2 - $
Double chain silicates, in which two simple chains are linked together by shared oxygen, can also be drawn. Amphiboles are another name for such compounds. Asbestos is a well-known example of a double chain silicate mineral. The general formula for the anions of double chain silicates is $\left( {{\text{S}}{{\text{i}}_4}{{\text{O}}_{11}}} \right)_{\text{n}}^{6{\text{ - }}}$.
e.g. Synthetic silicates $\left( {{\text{L}}{{\text{i}}_2}{\text{Si}}{{\text{O}}_3},{\text{N}}{{\text{a}}_2}{\text{Si}}{{\text{O}}_3}} \right)$, Spondumene $\left( {\operatorname{LiAl} {{\left( {{\text{Si}}{{\text{O}}_3}} \right)}_2}} \right)$,
Enstatite $\left( {{\text{MgSi}}{{\text{O}}_3}} \right)$, Diopside $\left( {{\text{CaMg}}{{\left( {{\text{Si}}{{\text{O}}_3}} \right)}_2}} \right)$, Tremolite $\left( {{\text{C}}{{\text{a}}_2}{\text{M}}{{\text{g}}_5}{{\left( {{\text{S}}{{\text{i}}_4}{{\text{O}}_{11}}} \right)}_2}{{({\text{OH}})}_2}} \right)$, etc.
Two dimensional sheet silicates in such silicates, Each tetrahedral shares three oxygen atoms with neighbouring${\text{SiO}}_4^4$ tetrahedrals. This sharing results in a two-dimensional sheet structure with a generic formula, $\left( {{\text{S}}{{\text{i}}_2}{{\text{O}}_5}} \right)_n^{22} - $such as Talc.$\left( {{\text{Mg}}{{\left( {{\text{S}}{{\text{i}}_2}{{\text{O}}_5}} \right)}_2}{\text{Mg}}{{({\text{OH}})}_2},\operatorname{Kaolin} {\text{A}}{{\text{l}}_2}{{({\text{OH}})}_4}\left( {{\text{S}}{{\text{i}}_2}{{\text{O}}_5}} \right)} \right.$
Three Dimensional Sheet Silicates:
All four oxygen atoms are shared by neighbouring${\text{SiO}}_4^4$ tetrahedral units in these silicates.
e.g. Quartz, Tridymite, Crystobalite, Feldspar, Zeolite and Ultramarines.
Silicones:
Silicones are synthetic organosilicon compounds with Si-O-Si bonds that hold repeating units of ${{\text{R}}_2}{\text{SiO}}$together. These compounds have an alkyl or aryl group in their general formula${\left( {{{\text{R}}_2}{\text{SiO}}} \right)_n}$. where ${\text{R}} = $ alkyl or aryl group.
The silicones are made by polymerizing alkyl or aryl substituted chlorosilanes after they have been hydrolysed. The following processes produce alkyl or aryl substituted chlorosilanes.
${\text{RCl + SiS}}\xrightarrow[{{\text{30}}{{\text{0}}^{\text{o}}}{\text{C}}}]{{{\text{Cu}}}}{{\text{R}}_{\text{3}}}{\text{SiCl + }}{{\text{R}}_{\text{2}}}{\text{SiC}}{{\text{l}}_{\text{2}}}{\text{ + RSiC}}{{\text{l}}_{\text{3}}}$
${\text{RMgCl}} + {\text{SiC}}{{\text{l}}_4}\xrightarrow{{}}{\text{RSiC}}{{\text{l}}_3} + {\text{MgCl}}$
$2{\text{RMgCl}} + {\text{SiC}}{{\text{l}}_4}\xrightarrow{{}}{{\text{R}}_2}{\text{SiC}}{{\text{l}}_2} + 2{\text{MgC}}{{\text{l}}_2};{\text{ }}3{\text{RMgCl}} + {\text{SiC}}{{\text{l}}_4}\xrightarrow{{}}{{\text{R}}_3}{\text{SiCl}} + 3{\text{MgCl}}$
The silane derivatives are hydrolysed during fractional distillation, and the resulting 'hydroxides' rapidly condense due to intermolecular water elimination. The amount of hydroxyl groups originally linked to the silicon atom determines the ultimate product:
Several molecules can be combined in this way to produce a long chain polymer with$ - {\text{OH}}$ groups at both ends. The following formula is used to denote such compounds.
A little amount of the monochlorosilane derivative is added to the hydrolysis mixture to terminate the polymer chain seen above.
Only the following chemicals can be used to make silicones.
${{\text{R}}_3}{\text{SiCl}}$
${{\text{R}}_2}{\text{SiC}}{{\text{l}}_2}$
${\text{RSiC}}{{\text{l}}_3}$
Silicones from the hydrolysis of ${\left( {{\text{C}}{{\text{H}}_3}} \right)_3}{\text{SiCl}}$
${\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}}{\text{SiCl}}\xrightarrow{{{{\text{H}}_{\text{2}}}{\text{O}}}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}}{\text{Si(OH)}}$
Silicones from the hydrolysis of a combination of ${\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}}{\text{SiCl}} \&{\left({{\text{C}}{{\text{H}}_{\text{3}}}}\right)_{\text{2}}}{\text{SiCl}}$
As is customary, the dichloro derivative will form a long chain polymer. However, the hydrolysis product of the mono-chloro derivative might stifle the development of this polymer at any point.
Silicones produced through trichloro derivative hydrolysis When a chemical like${\text{C}}{{\text{H}}_3}{\text{SiC}}{{\text{l}}_3}$ is hydrolyzed, it forms a complicated cross-linked polymer.
Silicones are water-repellent due to the hydrocarbon layer that runs along the silicon-oxygen chain. Silicones may be used to make products with the physical characteristics of oils, rubbers, and resins. Silicone fluids (such as those used in plane hydraulic systems) are thermally stable and their viscosity changes very little as a function of temperature; silicone rubbers, on the other hand, keep their flexibility at lower temperatures than regular rubber. Silicone varnishes are such good insulators and heat-resistant that using them to insulate wire allowed motors to run at over-loads that would have caused the insulation to catch fire. The discovery of silicones has opened up a whole new sector of science and technology, both civilian and military.
Tin and Lead:
Compounds of Tin:
Stannous Oxide $Sno$:
Preparation:
In the absence of air, stannous hydroxide, ${\text{Sn}}{({\text{OH}})_2}$is heated.
\[{\text{Sn}}{({\text{OH}})_2}\xrightarrow{{}}{\text{SnO}} + {{\text{H}}_2}{\text{O}} \uparrow \]
By heating stannous oxalate, ${\text{Sn}}{{\text{C}}_2}{{\text{O}}_4}$ in absence of air.
${\text{Sn}}{{\text{C}}_2}{{\text{O}}_4}\xrightarrow{{}}{\text{SnO}} + {\text{CO}} \uparrow + {\text{C}}{{\text{O}}_2}$
Properties:
${\text{SnO}}$ is an insoluble in water amphoteric dark grey or black solid oxide. It forms stannous salts when it dissolves in acids.
${\text{SnO}} + 2{\text{HCl}}\xrightarrow{{}}{\text{SnC}}{{\text{l}}_2} + {{\text{H}}_2}{\text{O}};{\text{ SnO}} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4}\xrightarrow{{}}{\text{SnS}}{{\text{O}}_4} + {{\text{H}}_2}{\text{O}}$
In a heated solution of ${\text{NaOH}}$, SnO dissolves to create (soluble) sodium stannite and water.
${\text{SnO}} + 2{\text{NaOH}}\xrightarrow{{}}{\text{N}}{{\text{a}}_2}{\text{Sn}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}}$
Only aqueous solutions are known to contain stannites. Stannites take oxygen from the air and oxidise to stannate, which is a naturally stable compound.
$2{\text{N}}{{\text{a}}_2}{\text{Sn}}{{\text{O}}_2} + {{\text{O}}_2}\xrightarrow{{}}2{\text{N}}{{\text{a}}_2}{\text{Sn}}{{\text{O}}_3}$
Uses:
It's used to make stannous chloride and stannous sulphate.
Stannous Chloride $(SnCl_{2}-2H_{2}O)$ :
It's a colourless solid that dissolves in water. Its solution becomes milky over a period of time owing to hydrolysis to $\operatorname{Sn} {({\text{OH}})_2}$ and HCl. Litmus finds the watery solution acidic. It's a powerful diluting agent. It's also soluble in ether and alcohol.
Preparation:
$Sn+2HCL(conc.)\rightarrow Sncl_{2}(aq)+H_{2}$
${\text{SnO}} + 2{\text{HCl}}\xrightarrow{{}}{\text{SnC}}{{\text{l}}_2}({\text{aq}}) + {{\text{H}}_2}{\text{O}}$
On crystallisation, the solution yields colourless crystals of ${\text{SnC}}{{\text{l}}_2} \cdot 2{{\text{H}}_2}{\text{O}}$.
Properties:
Reaction with ${\text{H}}{{\text{g}}_2}{\text{C}}{{\text{l}}_2}$ solution: When${\text{SnC}}{{\text{l}}_2}$ solution is added to an aqueous mercuric chloride solution, a silky white mercurous chloride ${\text{H}}{{\text{g}}_2}{\text{C}}{{\text{l}}_2}$ precipitate is produced, which becomes black when the mercury is reduced ${\text{H}}{{\text{g}}_2}{\text{C}}{{\text{l}}_2}$ further to black mercury.
$2{\text{HgC}}{{\text{l}}_2} + {\text{SnC}}{{\text{l}}_2}\xrightarrow{{}}{\text{H}}{{\text{g}}_2}{\text{C}}{{\text{l}}_2} \downarrow {\text{}} + {\text{SnC}}{{\text{l}}_4};{\text{ H}}{{\text{g}}_2}{\text{C}}{{\text{l}}_2} + {\text{SnC}}{{\text{l}}_2}\xrightarrow{{}}2{\text{Hg}} \downarrow {\text{}} + {\text{SnC}}{{\text{l}}_4}$
It reduces ferric chloride, ${\text{FeC}}{{\text{l}}_3}$ to ferrous chloride, $ + {\text{SnC}}{{\text{l}}_4}$${\text{FeC}}{{\text{l}}_{\text{2}}}{\text{.2FeC}}{{\text{l}}_{\text{3}}}\left( {{\text{brown solution}}} \right){\text{ + SnC}}{{\text{l}}_{\text{2}}}\xrightarrow{{}}{\text{2FeC}}{{\text{l}}_{\text{2}}}{\text{(colourless sol}}{\text{.) + SnC}}{{\text{l}}_{\text{4}}}$
It is hydrolyzed in water, resulting in a white precipitate of ${\text{Sn}}{({\text{OH}})_2}$ ${\text{SnC}}{{\text{l}}_2} + 2{{\text{H}}_2}{\text{O}} \Leftrightarrow {\text{Sn}}{({\text{OH}})_2}$ (white) $ \downarrow + 2{\text{HCl}}$
Its aqueous solution is acidic because it creates a weak basic and a strong acid. Hydrolysis can be avoided by adding concentrated${\text{HCl}}$ to it during the preparation procedure.
Uses:
It's utilised as a reducing agent in the dye business, as well as for mercuric salt testing and the production of various stannous compounds.
Stannic Oxide $SnO_{2}$:
Preparation:
By burning Sn in air
${\text{Sn}} + {{\text{O}}_2}\xrightarrow{{}}{\text{Sn}}{{\text{O}}_2}$
By heating Sn with concentrated ${\text{HN}}{{\text{O}}_3}$
${\text{Sn}} + 4{\text{HN}}{{\text{O}}_3}\xrightarrow{{}}{{\text{H}}_2}{\text{Sn}}{{\text{O}}_3} + 4{\text{N}}{{\text{O}}_2} \uparrow + {{\text{H}}_2}{\text{O}};{{\text{H}}_2}{\text{Sn}}{{\text{O}}_3}\xrightarrow{\Delta }{{\text{H}}_2}{\text{O}} \uparrow + {\text{Sn}}{{\text{O}}_2}$
Properties:
It's a white solid that won't dissolve in water. It has a low acidity. It forms stannic sulphate when dissolved in ${{\text{H}}_2}{\text{S}}{{\text{O}}_4}$r. It also dissolves in alkali metal stannate solution when mixed with conc. alkalies.
${\text{Sn}}{{\text{O}}_2} + 2{{\text{H}}_2}{\text{S}}{{\text{O}}_4}\xrightarrow{{}}{\text{Sn}}{\left( {{\text{S}}{{\text{O}}_4}} \right)_2} + 2{{\text{H}}_2}{\text{O}}$
${\text{Sn}}{{\text{O}}_{\text{2}}}{\text{ + 2NaOH}}\xrightarrow{{}}{\text{N}}{{\text{a}}_{\text{2}}}{\text{Sn}}{{\text{O}}_{\text{3}}}{\text{ }}\left( {{\text{sodium stannate}}} \right){\text{ + }}{{\text{H}}_{\text{2}}}{\text{O}}$
Stannic Chloride $SnCl_{4}$
Preparation:
By the action of ${\text{C}}{{\text{l}}_2}$ gas on heated ${\text{Sn}}$
${\text{Sn}} + 2{\text{C}}{{\text{l}}_2}\xrightarrow{{}}{\text{SnC}}{{\text{l}}_4}$
By the action of ${\text{C}}{{\text{l}}_2}$ on stannous chloride
${\text{SnC}}{{\text{l}}_2} + {\text{C}}{{\text{l}}_2}\xrightarrow{{}}{\text{SnC}}{{\text{l}}_4}$
Properties:
It's a colourless, flammable liquid. ${\text{Bp}} = 114^\circ {\text{C}}$. It has a covalent bond.
Action of Moisture It absorbs moisture and converts to hydrated stannic chlorides, ${\text{SnC}}{{\text{l}}_4} \cdot 3{{\text{H}}_2}{\text{O}},{\text{SnC}}{{\text{l}}_4} \cdot 5{{\text{H}}_2}{\text{O}},{\text{SnC}}{{\text{l}}_4} \cdot 6{{\text{H}}_2}{\text{O}}$, and ${\text{SnC}}{{\text{l}}_4} \cdot 8{{\text{H}}_2}{\text{O}} \cdot {\text{SnC}}{{\text{l}}_4} \cdot 5{{\text{H}}_2}{\text{O}}$ which is why it's called "butter of tin" or "oxymercurate of tin."
It is quickly hydrolyzed in water and generates a powerful acid ${\text{HCl}}$. As a result, litmus finds its aqueous solution acidic. It hydrolyzes at a faster rate than ${\text{SnC}}{{\text{l}}_2}$
${\text{SnC}}{{\text{l}}_{\text{4}}}{\text{ + 4}}{{\text{H}}_{\text{2}}}{\text{O}}\xrightarrow{{}}{{\text{H}}_{\text{4}}}{\text{Sn}}{{\text{O}}_{\text{4}}}{\text{ + 4HCl}}$
${\text{SnC}}{{\text{l}}_4}$ is a Lewis acid. Hence it has a tendency to accept lone pair of electrons from ${\text{N}}{{\text{H}}_3},{\text{P}}{{\text{H}}_3}$ etc. and form adducts such as ${\text{SnC}}{{\text{l}}_4} \cdot 4{\text{N}}{{\text{H}}_3}$
It dissolves in concentrated ${\text{HCl}}$ forming ${{\text{H}}_2}{\text{SnC}}{{\text{l}}_6}$ and in presence of ammonium chloride, it forms ammonium salts of this acid.
${\text{SnC}}{{\text{l}}_4} + 2{\text{HCl}}\xrightarrow{{}}{{\text{H}}_2}{\text{SnC}}{{\text{l}}_6}$
${\text{SnC}}{{\text{l}}_4} + 2{\text{N}}{{\text{H}}_4}{\text{Cl}}\xrightarrow{{}}{\left( {{\text{N}}{{\text{H}}_4}} \right)_2}{\text{SnC}}{{\text{l}}_6}$
Uses:
For the preparation of stannic compounds.
Compounds Of Lead:
Litharge$(PbO)$:
${\text{PbO}}$ is obtained by heating ${\text{Pb}}$ at ${180^\circ }{\text{C}}$. It is an unstable yellow compound. $2\;{\text{Pb}} + {{\text{O}}_2}\xrightarrow{\Delta }2{\text{PbO}}$
It's an amphoteric oxide that dissolves in both acids and alkalis. ${\text{PbO}} + 2{\text{HN}}{{\text{O}}_3}\xrightarrow{\Delta }{\text{Pb}}{\left( {{\text{N}}{{\text{O}}_3}} \right)_2} + {{\text{H}}_2}{\text{O}};{\text{ PbO}} + 2{\text{NaOH}}\xrightarrow{\Delta }{\text{N}}{{\text{a}}_2}{\text{Pb}}{{\text{O}}_2} + {\text{ }}{{\text{H}}_{\text{2}}}{\text{O}}$
It's utilised in the rubber sector, as well as flint glasses, enamels, and storage batteries.
Lead Dioxide$(PbO^{2})$ :
The fact that ${{\text{H}}_2}{{\text{O}}_2}$ is not liberated by dilute ${\text{HCl}}$ suggests that the preceding formula is correct (It is a dioxide not a peroxide)
Preparation:
${\text{PbO + NaOCl}}\xrightarrow{{\Delta}}{\text{ Pb}}{{\text{O}}_{\text{2}}}{\text{(insoluble) + NaCl}}$
${\text{P}}{{\text{b}}_{\text{3}}}{{\text{O}}_{\text{4}}}{\text{ + 4HN}}{{\text{O}}_{\text{3}}}{\text{(dilute)}}\xrightarrow{{}}{\text{2Pb}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}}{\text{ + Pb}}{{\text{O}}_{\text{2}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}}$
Properties:
It's an insoluble powder with a chocolate color. It produces monoxide when heated at ${440^\circ }{\text{C}}$:
$2{\text{Pb}}{{\text{O}}_2}\xrightarrow{{{{440}^\circ }{\text{C}}}}2{\text{PbO}} + {{\text{O}}_2}$
It oxidizes ${\text{HCl}}$ to ${\text{C}}{{\text{l}}_2}$ :
${\text{Pb}}{{\text{O}}_2} + 4{\text{HCl}}\xrightarrow{{}}{\text{PbC}}{{\text{l}}_2} + 2{{\text{H}}_2}{\text{O}} + {\text{C}}{{\text{l}}_2} \uparrow $
It dissolves in conc. ${\text{NaOH}}$ solution:
${\text{Pb}}{{\text{O}}_{\text{2}}}{\text{ + 2NaOH}}\xrightarrow{{}}{\text{N}}{{\text{a}}_{\text{2}}}{\text{Pb}}{{\text{O}}_{\text{3}}}{\text{ (sodiumplumbate) + }}{{\text{H}}_{\text{2}}}{\text{O}}$
It oxidises Mn salt to permanganic acid:
$2{\text{MnS}}{{\text{O}}_4} + 5{\text{Pb}}{{\text{O}}_2} + 6{\text{HN}}{{\text{O}}_3}\xrightarrow{{}}2{\text{HMn}}{{\text{O}}_4} + 2{\text{PbS}}{{\text{O}}_4} + 3\;{\text{Pb}}{\left( {{\text{N}}{{\text{O}}_3}} \right)_2} + 2{{\text{H}}_2}{\text{O}}$
It reacts with ${\text{S}}{{\text{O}}_2}$ at red heat to form lead sulphate:
${\text{Pb}}{{\text{O}}_2} + {\text{S}}{{\text{O}}_2}\xrightarrow{\Delta }{\text{PbS}}{{\text{O}}_4}$
It reacts with conc. ${\text{HN}}{{\text{O}}_3}$ to evolve oxygen gas. ${\text{Pb}}{{\text{O}}_2} + 2{\text{HN}}{{\text{O}}_3}\xrightarrow{{}}{\text{Pb}}{\left( {{\text{N}}{{\text{O}}_3}} \right)_2} + 1/2{{\text{O}}_2} + {{\text{H}}_2}{\text{O}}$
Uses:
It's used in the match business to make the igniting surface of match boxes and to make match powder ${\text{KMn}}{{\text{O}}_4}$.
Red Lead$((PbO^{4}))$:
Preparation:
It is prepared by heating ${\text{PbO}}$ at ${450^\circ }{\text{C}}$ for a long time.
${\text{6PbO + }}{{\text{O}}_{\text{2}}}\xrightarrow{{{\text{45}}{{\text{0}}^{\text{o}}}{\text{C}}}}{\text{2P}}{{\text{b}}_{\text{3}}}{{\text{O}}_{\text{4}}}$
Properties:
It is a red powder that is insoluble in water but produces a red precipitate of ${\text{Pb}}{{\text{O}}_2}$when heated with conc. ${\text{HN}}{{\text{O}}_3}$
${\text{P}}{{\text{b}}_3}{{\text{O}}_4} + 4{\text{HN}}{{\text{O}}_3}\xrightarrow{{}}2\;{\text{Pb}}{\left( {{\text{N}}{{\text{O}}_3}} \right)_2} + {\text{Pb}}{{\text{O}}_2} \downarrow + 6{{\text{H}}_2}{\text{O}}$
When heated above ${550^\circ }{\text{C}}$, it undergoes decomposition into ${\text{PbO}}$ and liberate oxygen gas.
${\text{P}}{{\text{b}}_3}{{\text{O}}_4}\xrightarrow{\Delta }6{\text{PbO}} + {\text{O}}2 \uparrow $
It oxidizes conc. ${\text{HCl}}$ to chlorine
${\text{P}}{{\text{b}}_3}{{\text{O}}_4} + 8{\text{HCl}}\xrightarrow{{}}3{\text{PbC}}{{\text{l}}_2} + 4{{\text{H}}_2}{\text{O}} + {\text{C}}{{\text{l}}_2} \uparrow $
When heated with conc. ${{\text{H}}_2}{\text{S}}{{\text{O}}_4}$ it evolves oxygen $2\;{\text{P}}{{\text{b}}_3}{{\text{O}}_4} + 6{{\text{H}}_2}{\text{S}}{{\text{O}}_4}\xrightarrow{{}}6{\text{PbS}}{{\text{O}}_4} + 6{{\text{H}}_2}{\text{O}} + {{\text{O}}_2} \uparrow $
Uses:
It is employed as an oxidising agent, in the production of red paint, in the production of special lead cement, and in the production of flint glass.
Lead Chloride $(PbCl^{2})$ :
Preparation:
${\text{Pb}}{\left( {{\text{N}}{{\text{O}}_3}} \right)_2} + 2{\text{HCl}}\xrightarrow{{}}{\text{PbC}}{{\text{l}}_2} \downarrow + 2{\text{HN}}{{\text{O}}_3}$
\[{\text{Pb}}{\left( {{\text{N}}{{\text{O}}_3}} \right)_2} + 2{\text{NaCl}}\xrightarrow{{}}{\text{PbC}}{{\text{l}}_2} \downarrow + 2{\text{NaN}}{{\text{O}}_3}\]
${\text{Pb}}{\left( {{\text{C}}{{\text{H}}_3}{\text{COO}}} \right)_2} + 2{\text{HCl}}\xrightarrow{{}}{\text{PbC}}{{\text{l}}_2} \downarrow + 2{\text{C}}{{\text{H}}_3}{\text{COOH}}$
\[{\text{PbO}} + 2{\text{HCl}}\xrightarrow{{}}{\text{PbC}}{{\text{l}}_2} \downarrow + {{\text{H}}_2}{\text{O}}\]
${\text{Pb}}{({\text{OH}})_2} + 2{\text{HCl}}\xrightarrow{{}}{\text{PbC}}{{\text{l}}_2} \downarrow + 2{{\text{H}}_2}{\text{O}}$
${\text{Pb}}{({\text{OH}})_2} \cdot {\text{PbC}}{{\text{O}}_3} + 4{\text{HCl}}\xrightarrow{{}}2{\text{PbC}}{{\text{l}}_2} \downarrow + {\text{C}}{{\text{O}}_2} \uparrow + 3{{\text{H}}_2}{\text{O}}$
Properties:
It's a crystalline white substance that's insoluble in cold water but soluble in hot water. It forms a complex ion when dissolved in strong HCl.
\[{\text{2HCl + PbC}}{{\text{l}}_{\text{2}}}{\;}\overset {} \leftrightarrows {\;}{{\text{H}}_{\text{2}}}{\text{PbC}}{{\text{l}}_{\text{4}}}\left( {{\text{chloroplumbous acid}}} \right)\]
Uses:
It's utilised in the production of paint pigments.
Lead Tetrachloride $(PbCl^{4})$ :
Preparation:
The following procedures are used to make it:
By dissolving ${\text{Pb}}{{\text{O}}_2}$ in cold conc. HCl
${\text{Pb}}{{\text{O}}_2} + 4{\text{HCl}}\xrightarrow{{}}{\text{PbC}}{{\text{l}}_4} + 2{{\text{H}}_2}{\text{O}}$
${\text{PbCl}}_4^2$ dissolves in excess of ${\text{HCl}}$ to form a stable solution of ${{\text{H}}_2}{\text{PbC}}{{\text{l}}_6}$ ${\text{PbCl}}_4^ + + 2{\text{HCl}}\xrightarrow{{}}{{\text{H}}_2}{\text{PbC}}{{\text{l}}_6}$
When ${\text{N}}{{\text{H}}_4}{\text{Cl}}$ is introduced to a chloroplumbic acid solution, a yellow precipitate of ammonium chloroplumbate is produced.
${{\text{H}}_2}{\text{PbC}}{{\text{l}}_6} + 2{\text{N}}{{\text{H}}_4}{\text{Cl}}\xrightarrow{{}}{\left( {{\text{N}}{{\text{H}}_4}} \right)_2}{\text{PbC}}{{\text{l}}_6} \downarrow + 2{\text{HCl}}$
Lead tetrachloride is produced when crystals of ammonium chloroplumbate are introduced to ice cold conc. ${{\text{H}}_2}{\text{S}}{{\text{O}}_4}$ and separate as a yellow oily liquid.
${\left( {{\text{N}}{{\text{H}}_4}} \right)_2}{\text{PbC}}{{\text{l}}_6} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4}\xrightarrow{{}}{\text{PbC}}{{\text{l}}_4} + {\left( {{\text{N}}{{\text{H}}_4}} \right)_2}{\text{S}}{{\text{O}}_4} + 2{\text{HCl}}$
By the action of ${\text{C}}{{\text{l}}_2}$ on a solution of ${\text{PbC}}{{\text{l}}_2}$ in conc. ${\text{HCl}}$
${\text{PbC}}{{\text{l}}_2} + {\text{C}}{{\text{l}}_2}\xrightarrow{{}}{\text{PbC}}{{\text{l}}_4}$
Properties:
It's a yellow oily liquid that freezes at $ - {10^\circ }{\text{C}}$ and dissolves in organic solvents like ethanol and benzene.
Precipitation of ${\text{Pb}}{{\text{O}}_2}$ is formed by rapid hydrolysis with water.
\[{\text{PbC}}{{\text{l}}_4} + 2{{\text{H}}_2}{\text{O}}\xrightarrow{{}}{\text{Pb}}{{\text{O}}_2} \downarrow + 4{\text{HCl}}\]
Uses: It's utilised in the production of stannic compounds.
About The p-Block Elements
The p-block elements are the interesting group of elements found usually on the right side of the periodic table from column number 3A to 8A (including the segments 13-18). A few of the p-block elements include noble gases, metalloids, halogens, and metals. Therefore, in chemistry, class 11, the topic p-Block elements are dealt with in more detail. The students need to learn the anomalous properties, general trends, allotropes, and important compounds of p-block elements.
The p-block elements topics deal with the elements present in the p-block of the modern periodic table. Also, there are variations in the properties of the p-block elements due to the influence of d-block and f-block electrons present in the heavier elements of the inner core.
Also, once this chapter is done, students can learn about the trends in the chemistry of p-block elements. They will also know about the trends in physical and chemical properties of group 13 and group 14 elements and can know the anomalous behavior of Boron and Carbon.
Even they will describe the allotropic forms of Carbon and get an idea about the compounds of Carbon, Silicon, and Boron. A few of the concepts described in group 13 elements are the boron family, the atomic radii, electronegativity, physical and chemical properties, some important compounds of boron, borax, and more.
In addition to these, the concepts used in group 14 elements are carbon family, electronic configuration, covalent radius, physical and chemical properties, electronegativity, allotropes of carbon, diamond, graphite, and more.
Sub-Topics Covered Under The p-Block Elements
The chemistry class 11, Chapter 11 of, The p-block elements falls under the part of unit 11. The total weight of the unit is 8, 9, 10, 11, and carries 16 marks. The student, by studying this chapter, can become 100 percent accurate on the NCERT solutions for class 11 Chemistry at Vedantu, solved by our subject experts.
Let us look at the topics and subtopics in the p-block elements chapter below:
Group 13 Elements - The Boron Family
Ionization Enthalpy
Atomic Radii
Electronegativity
Electronic Configuration
Physical and Chemical Properties
Important Trends & Anomalous Properties of Boron
Some Important Compounds of Boron
Borax
Diborane, B2H6
Orthoboric Acid
Uses of Boron & Aluminium & Their Compounds
Group 14 Elements: The Carbon Family
Covalent Radius
Electronic Configuration
Ionization Enthalpy
Physical and Chemical properties
Electronegativity
Important Trends & Anomalous Behaviour of Carbon
Allotropes of Carbon
Graphite
Diamond
Fullerenes
Uses of Carbon
Some Important Compounds of Carbon & Silicon
Carbon Monoxide and
Carbon Dioxide
Silicon Dioxide, Sio2
Silicates
Silicones
Zeolites
Why Choose Vedantu?
As India’s largest online tutoring platform, Vedantu is the best in providing the required information to the students concerning their academics. Let us look at some of the benefits of Vedantu.
Students can find their academic stuff such as “Revision Notes, Previous Year Question Papers, Last 5 Years Question Papers, Tips and Tricks to crack the exams,“ and more
All these stuff can also be downloaded from the official website of Vedantu, vedantu.com, where the students can use these files a day or twice before the exam comes and can prepare more easily
All the required documents can be availed at completely free of cost
In addition to these, they can also get the proper assistance concerning their syllabus, doubts on any topics, and more related
They can get the topics from many of the subjects from all over the country
Students can download many topics by applying the keywords such as alcohols phenols and ethers class 12 notes, class 12 chemistry chapter 11 notes, chapter 11 chemistry class 12 notes, class 12 chemistry chapter alcohol phenol and ether notes, Notes of chapter 11 chemistry class 12, class 12 chemistry ch 11 notes, Notes of chemistry class 12 alcohols phenols and ethers
Importance of a Revision Notes
A revision notes is the one which can be either prepared by the students or by other third-party providers and is so important. It is used to note the important points, formulas, points to be remembered. Also, it can be very helpful during the exams, where the students can make the most of it by carrying easily because it is handy.
FAQs on CBSE Notes Class 11 Chemistry Chapter 11 - The P Block Elements - 2025-26
1. What is a quick summary of p-block elements as covered in Class 11?
A summary of p-block elements for Class 11 focuses on elements where the last electron enters the outermost p-orbital. The curriculum covers Group 13 (the Boron family) and Group 14 (the Carbon family). Their general valence shell electronic configuration is ns²np¹⁻². These groups are notable for containing a diverse mix of metals, metalloids, and non-metals, showing a wide range of properties.
2. How can I structure my revision for the key topics in Chapter 11, The p-Block Elements?
For an effective revision of this chapter, you should cover the topics in a logical sequence. Start with the general trends, then move to specifics:
- Begin with the periodic trends in Group 13 and 14, such as atomic radii, ionisation enthalpy, and electronegativity.
- Focus on the anomalous properties of the first elements, Boron and Carbon.
- Revise key compounds of Boron, including borax, boric acid, and diborane.
- Study the allotropes of Carbon (diamond, graphite, fullerenes).
- Conclude with important compounds of Carbon (CO, CO₂) and Silicon (silicones, silicates, zeolites).
3. What are the general properties to remember for the p-block elements?
The key property to remember for p-block elements is their chemical diversity. The block includes non-metals like Carbon, metalloids like Silicon and Boron, and metals like Aluminium and Tin. This results in a wide variation of physical and chemical properties. For instance, non-metals are generally insulators with low boiling points, while the metals are good conductors with higher melting points.
4. How does the inert pair effect explain stability trends in p-block elements?
The inert pair effect is a crucial concept for revision. It describes the reluctance of the two s-electrons in the valence shell to participate in chemical bonding. This effect becomes more significant as you move down a group. For example, in Group 14, Carbon and Silicon primarily show a +4 oxidation state, but as we move down to Lead (Pb), the +2 oxidation state becomes more stable than the +4 state due to this effect.
5. Why does Boron exhibit anomalous behaviour compared to other Group 13 elements?
Boron's unique behaviour is a key concept to revise. Its anomalous properties are primarily due to three factors:
- Its extremely small atomic size.
- Its very high ionisation enthalpy.
- The absence of d-orbitals in its valence shell, limiting its covalency.
These factors cause Boron to be a hard, non-metallic solid that forms covalent compounds, unlike the other elements in its group which are metallic.
6. What is the main structural difference between diamond and graphite for a quick recap?
The fundamental structural difference lies in the hybridisation and bonding of carbon atoms. In diamond, each carbon atom is sp³ hybridised and bonded to four other carbons in a rigid tetrahedral 3D network. In graphite, each carbon is sp² hybridised and bonded to three others, forming flat hexagonal layers that can slide over each other. This is why diamond is extremely hard, while graphite is soft and used as a lubricant.
7. How do the three main allotropes of carbon differ?
The allotropes of carbon—diamond, graphite, and fullerenes—differ in the structural arrangement of their atoms. Diamond features a tetrahedral, 3D lattice making it the hardest known substance. Graphite has a layered, planar structure, making it soft and a good electrical conductor. Fullerenes, like C₆₀, are cage-like molecules with a spherical shape, often called 'buckyballs'.
8. What is the core difference between silicates and silicones?
While both are silicon compounds, their structures and properties are very different. Silicates are minerals containing basic SiO₄⁴⁻ tetrahedral units, forming the basis of rocks and glass. Silicones are synthetic polymers with an alternating silicon-oxygen backbone (-Si-O-Si-), with organic side groups attached to the silicon atoms. Silicones are prized for their thermal stability and water-repellent nature.
9. Why is carbon monoxide (CO) so much more toxic than carbon dioxide (CO₂)?
Carbon monoxide (CO) is highly toxic because it binds to the haemoglobin in red blood cells with an affinity about 250-300 times greater than that of oxygen. This forms a stable complex called carboxyhaemoglobin, which severely reduces the blood's capacity to transport oxygen to vital organs. Carbon dioxide does not bind in this manner, making CO exceptionally dangerous even at low concentrations.
10. What are the s- and p-block elements collectively called?
The elements belonging to the s-block and p-block of the periodic table are collectively known as the representative elements or main group elements. They are called this because they display a wide and representative range of physical and chemical properties. As per the CBSE 2025-26 syllabus, understanding this classification is essential context for the chapter.

























