Chemistry Notes for Chapter 10 The S Block Elements Class 11 - FREE PDF Download
In Cbse Class 11 Chemistry Notes Chapter 10 The S Block Elements, you’ll dive into the world of alkali and alkaline earth metals—like sodium, potassium, magnesium, and calcium. These elements are at the very start of the periodic table and come up often in CBSE exams, so understanding them can boost your confidence. This chapter will help you learn their properties, reactions, uses, and the special tricks to remember them easily.
To make your study smart and effective, don’t forget to check the Class 11 Chemistry Syllabus for all updated topics covered in CBSE. Learning this chapter becomes simple when you have our Class 11 Chemistry Revision Notes as your guide. Vedantu’s notes break down tough points, clear your doubts, and help you revise quickly for exams.
This chapter is quite important because questions from the s-block elements appear regularly in board papers. Mastering it can really help you score well in your Chemistry exams.
The s-Block Elements Class 11 Notes Chemistry - Basic Subjective Questions
Section – A (1 Mark Question
1. Melting and boiling points of alkali metals is low. Explain?
Ans. The melting and boiling points of the alkali metals are low indicating weak metallic bonding due to the presence of only a single valence electron in them.
2. Explain the diagonal relationship in periodic table.
Ans. The diagonal relationship is due to the similarity in ionic sizes and/or charge/radius ratio of the elements.
3. Why are lithium compounds soluble in organic solvents?
Ans. Due to high polarizing power, there is increased covalent character of lithium compounds which is responsible for their solubility in organic solvents.
4. By which process we can prepapre sodium carbonate?
Ans. Sodium carbonate is generally prepared by Solvay’s process.
5. What is the formula of soda ash?
Ans. Na2CO3 is the formaula of soda ash.
6. Why do alkaline earth metals have low ionization enthalpy?
Ans. The alkaline earth metals have low ionization enthalpies due to fairly large size of atoms.
7. What is the nature of oxide formed by Be?
Ans. BeO is covalent and amphoteric while oxides of other elements are ionic and basic in nature.
8. Beryllium show similarities with Al. Why?
Ans. Because of their similarity in charge/radius ratios.
(Be2+,2/31 = 0.064 and Al3+,3/50 = 0.66)
9. What happens when gypsum is heated to 390K?
Ans. When we heat gypsum at 390K ,Plaster of parts will form.
$2\text{CaSO}_4.2\text{H}_2\text{O}\xrightarrow[]{\text{390K}}(\text{CaSO}_4)\text{H}_2\text{O}+3\text{H}_2\text{O}$
10. Whyanhydrous calcium sulphate cannot be used as plaster of Paris?
Ans. Because it does not have the ability to set like plaster of Paris.
Section – B (2 Marks Questions)
11. Why metals like potassium and sodium cannot be extracted by reduction of their oxides by carbon?
Ans. Potassium and sodium are strong electropositive metals and have great affinity for oxygen than that of carbon. Hence they cannot be extracted from their oxides by reduction with carbon.
12. Complete the reactions and balance them.
(a) Na2O2 and water
(b) KO2 and water
Ans. (a)2Na2O2 + 2H2O → 4NaOH + O2
(b) 2KO2 + 2H2O → 2KOH + H2O + O2
13. Potassium carbonate can not be prepared by the SOLVAY process. Give reason.
Ans. Potassium carbonate cannot be prepared by the SOLVAY process because potassium bicarbonate (KHCO3) is highly soluble in water, unlike NaHCO3 which was separated as crystals. Due to its high solubility KHCO3 cannot be precipitated by the addition of ammonium bicarbonate to a saturated solution of KCl.
14. Explain what happens when
(i) Sodium hydrogen carbonate is heated.
(ii) Sodium with mercury reacts with water.
Ans. (i) $\underset{\text{Sodium hydrogen carbonate}}{2\text{NaHCO}_3}\xrightarrow[]{\text{Heat}}\underset{\text{Sodium carbonate}}{\text{Na}_2\text{CO}_3}+\text{H}_2\text{O}+\text{CO}_2\uparrow$
(ii) 2Na-Hg+2H2O→2NaOH+H2↑+2Hg
15. S-block elements never occur in free state. Explain. What are their usual modes of occurrence?
Ans. The metals (Alkali & alkaline earth metals) are highly reactive because of their low ionization energy. They are highly electropositive forming positive ions. So, they are never found in a free state. They are widely distributed in nature in a combined state. They occur in the earth’s crust in the form of oxides, chlorides, silicates & carbonates.
16. Why second ionization enthalpy of Calcium is more than the first? How is that calcium forms CaCl2 and not CaCl . Explain
Ans. The higher value of second ionization enthalpy is more than compensated by the higher enthalpy of hydration of Ca2+. Therefore, formation of CaCl2 becomes more favourable than CaCl energetically.
17. Solubility of alkaline metal hydroxides increases down the group . Explain.
Ans. If the anion and the cation are of comparable size, the cationic radius will influence the lattice energy. Since lattice energy decreases much more than the hydration energy with increasing ionic ionic size, solubility will increases as we go down the group. This is the case of alkaline earth metal hydroxides.
18. Solution of Na2CO3 is alkaline. Give reason.
Ans. The solution of Na2CO3 is alkaline in nature because when Na2CO3 is treated with water, it gets hydrolysed to form an alkaline solution:
CO32– + H2O → HCO3– + OH–
19. Discuss the various reactions that occur in the solvay process
Ans. 2NH3 + H2O + CO2→ (NH4)2CO3
(NH4)CO3 + CO2 + H2O → 2NH4HCO3
NH4HCO3 + NaCl → NH4Cl + NaHCO3
2NaHCO3→ Na2CO3 + CO2 + H2O
20. Give reason:
Lithium halides are covalent in nature.
Ans. Lithium halides are covalent because of the high polarization capability of lithium ion. The Li+ ion is very small in size and has high tendency to distort electron cloud around the negative halide ion.
PDF Summary - Class 11 Chemistry The s-Block Elements Notes (Chapter 12)
Alkali Metals (Group 1)
They have $n{s^1}$ electronic configuration and are highly reactive metals
Elements | Atomic Number | Electronic Configuration |
Lithium | 3 | $[He]\;2{s^1}$ |
Sodium | 11 | $[Ne]\;3{s^1}$ |
Potassium | 19 | $[Ar]\;4{s^1}$ |
Rubidium | 37 | $[Kr]\;5{s^1}$ |
Cesium | 55 | $[Xe]\;6{s^1}$ |
Francium | 87 | $[Rn]\;7{s^1}$ |
Physical Properties
Atomic Size
In their respective times, the atoms are at their largest. As you progress through the group, the atomic size grows larger.
Oxidation State
Group 1 elements has +1 oxidation state.
Density
Alkali metals have low density due to their large size.
$Density = \dfrac{{Atomic\;mass}}{{Atomic\;volume}}$
The group's atomic weight increases from $Li$ to $Cs$ , as does its volume, but the atomic weight increase outweighs the volume increase. As a result, density rises from $Li$ to $Cs$
Exception: Density of sodium is more than that of potassium
Order: $Li < K < Na < Rb < Cs$
Nature of Bonds
When the values of electronegativity are low, they combine with other elements to form Ionic bonds.
Ionization Energy
The atoms in this group have lower initial ionisation energy than any other group in the periodic table. Because atoms are so big, the outer electron is only weakly bound by the nucleus, resulting in a low ionisation energy. As you move down the group, the ionisation energy drops.
Flame Test
When alkali metals are burned on a flame, the electrons in the valence shell migrate from a lower energy level to a higher energy level due to heat absorption from the flame. When they return to their original condition, they release the additional energy in the form of visible light, which gives the flame colour.
Element | Colour |
$Li$ | Red |
$Na$ | Golden yellow |
$K$ | Violet |
$Rb$ | Red Violet |
$Cs$ | Blue |
Standard Oxidation Potential
The electrode potential of a metal in water is a measurement of its tendency to donate electrons. The standard electrode potential is defined as the concentration of metal ions being equal to one. Lithium has the largest ionisation potential, but due to its high hydration energy, it also has the highest electrode potential.
Hydration of Ions
The ions have a lot of water in them. The degree of hydration is proportional to the size of the ion. As a result, from Li+ to Cs+, the degree of hydration falls. As a result, electrical conductivity diminishes as hydration increases.
Lattice Energy
Ionic solids are alkali metal salts. The lattice energy of alkali metal salts with a common anion drops as one moves down the group.
Solubility in Liquid Ammonia
$M + nN{H_3} \to {[M{(N{H_3})_x}]^ + } + {e^ - }{(N{H_3})_y}$
$(n = x + y)$
The major species found in solvated metal ions and solvated electrons in dilute alkali metal solutions in liquid ammonia are solvated metal ions and solvated electrons. The colour diminishes until it disappears if the blue solution is left to stand due to the creation of metal amide. Because of the presence of solvated electrons, metal solutions in liquid conduct electricity. Because they include free electrons, the dilute solutions are paramagnetic.
Electronegativity Values
The electronegativity values are small which decrease from lithium to cesium.
Reactivity
The reactivity of alkali metals goes on increasing in the following order: $Li < Na < K < Rb < Cs$
Colourless and Diamagnetic Ions
The number of unpaired electrons present in an ion determines whether the ion is colourless or coloured. If an anion has unpaired electrons, these electrons can be stimulated by light energy and subsequently return to the ground state to show colour. Unpaired electron ions have magnetic properties, while paired electron ions cancel out each other's magnetic fields. Diamagnetic ions are such ions. The presence of unpaired electrons causes super oxides to be para magnetic and coloured.
Melting and Boiling Point
The cohesive energy is the force that holds the atoms or ions in a solid together. The cohesive energy is proportional to the number of electrons capable of bonding. Alkali metals contain only one valence electron that participates in bonding, and the outer bonding electron is big and diffuse, therefore the cohesive force reduces as the group gets smaller. As the atoms get bigger as you go down the group, the bonds get weaker, the cohesive energy drops, and the metal gets softer. As a result, the melting point drops as the group progresses. The boiling point also reduces the size of the group.
Chemical Properties
Some Common Reactions of Group 1 Metals
Reaction | Comment |
$M + {H_2}O \to MOH + {H_2}$ | Hydroxides are strongest base known. |
$Li + {O_2} \to L{i_2}O$ | Monoxide formed by lithium and to a small extent by sodium. |
$Na + {O_2} \to N{a_2}{O_2}$ | Peroxide formed by sodium and to a small extent by lithium. |
$K + {O_2} \to K{O_2}$ | Superoxide formed by potassium, rubidium and cesium. |
$M + {H_2} \to MH$ | Ionic salt like hydrides. |
$Li + {N_2} \to L{i_3}N$ | Nitride formed only by lithium |
$M + S \to {M_2}S$ | All metals form sulphides. |
$M + {X_2} \to MX$ | All metals form halides. |
$M + N{H_3} \to MN{H_2}$ | All the metals form amides. |
Reaction with Air
Group 1 elements are very reactive and tarnish quickly when exposed to air.
These metals form alkaline carbonates in moist air.
$2Na + {O_2} \to 2N{a_2}O$
$N{a_2}O + {H_2}O \to 2NaOH$
$2NaOH + C{O_2} \to N{a_2}C{O_3} + {H_2}O$
Reaction with ${O_2}$
Lithium forms $L{i_2}O$ , sodium forms two types of oxide $({M_2}O,\;{M_2}{O_2})$ and potassium, rubidium and cesium form superoxide $(M{O_2})$ .
Basic Nature, Ionic Nature of the Oxides
Because the size of the cation increases, the basic nature of oxides changes from lithium to cesium.
From lithium to cesium, the cation size increases. The ionic nature of these oxides rises from lithium to cesium, according to Fajan's Rule.
As the ionic nature of these metal oxides changes, solubility in water increases from lithium to cesium oxides.
Reaction with water
Group 1 metals react with water and liberates hydrogen and thus hydroxides are formed.
$2 \mathrm{Li}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow 2 \mathrm{LiOH}+\mathrm{H}_{2}$
$2 \mathrm{Na}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow 2 \mathrm{NaOH}+\mathrm{H}_{2}$
$2 \mathrm{~K}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow 2 \mathrm{KOH}+\mathrm{H}_{2}$
Reaction with Hydrogen
Group 1 metals react with hydrogen and forms ionic hydrides. Thermal stability of $\mathrm{LiH}$ is high.
Stability of hydrides is in the order:
$\mathrm{LiH}>\mathrm{NaH}>\mathrm{KH}>\mathrm{RbH}>\mathrm{CsH}$
Reaction with Dilute acids
These metals react quickly with dilute acids due to their alkaline nature, and the rate of reaction increases from lithium to cesium as the basic character increases.
Compounds of Alkali Metal
Hydroxides
Caustic soda is another name for sodium hydroxide. Because of its corrosive qualities, potassium hydroxide is known as caustic potash. The strongest base in aqueous solution is caustic alkali. The solubility of hydroxides rises as they progress through the group.
The bases react with acids to form salt and water
$\mathrm{KOH}+\mathrm{HCl} \rightarrow \mathrm{KCl}+\mathrm{H}_{2} \mathrm{O}$
$\mathrm{NaOH}+\mathrm{HCl} \rightarrow \mathrm{NaCl}+\mathrm{H}_{2} \mathrm{O}$
$2 \mathrm{NaOH}+\mathrm{CO}_{2} \rightarrow \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}$
Ammonia is liberated by the bases from ammonium salts
$\mathrm{NaOH}+\mathrm{NH}_{4} \mathrm{Cl} \rightarrow \mathrm{NH}_{3}+\mathrm{NaCl}+\mathrm{H}_{2} \mathrm{O}$
$\mathrm{KOH}+\mathrm{NH}_{4} \mathrm{Cl} \rightarrow \mathrm{NH}_{3}+\mathrm{KCl}+\mathrm{H}_{2} \mathrm{O}$
In all of its reactions, potassium hydroxide is similar to sodium hydroxide. However, because potassium hydroxide is so much more expensive, it is rarely utilised. However, because potassium hydroxide is more soluble in alcohol, the equilibrium produces $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{O}^{-}$ ions.
$\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}+\mathrm{OH}^{-} \rightleftharpoons \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{O}^{-}+\mathrm{H}_{2} \mathrm{O}$
Oxides, Peroxides and Superoxides
Normal oxides – monoxide: Ionic monoxides are present. They are very basic oxides that create strong bases when they react with water.
$\mathrm{Na}_{2} \mathrm{O}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{NaOH}$
$\mathrm{K}_{2} \mathrm{O}+\mathrm{H}_{3} \mathrm{O} \rightarrow \mathrm{KOH}$
Peroxides
Preparation:
$2 \mathrm{Na}+\mathrm{O}_{2}(\mathrm{excess}) \underset{\longrightarrow}{300^{\circ} \mathrm{C}}{\longrightarrow} \mathrm{Na}_{2} \mathrm{O}_{2}$
$2 \mathrm{Na}_{2} \mathrm{O} \quad{ }^{7400^{\circ} \mathrm{C}}{\longrightarrow} \mathrm{Na}_{2} \mathrm{O}_{2}+\mathrm{Na}$ (vapour)
Properties
$\mathrm{Na}_{2} \mathrm{O}_{2}+\mathrm{H}_{2} \mathrm{SO}_{4}(\mathrm{dil}) \rightarrow \mathrm{Na}_{2} \mathrm{SO}_{4}+\mathrm{H}_{2} \mathrm{O}_{2}$
$\mathrm{Na}_{2} \mathrm{O}_{2}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow 2 \mathrm{NaOH}+\mathrm{H}_{2} \mathrm{O}_{2}$
$\mathrm{Na}_{2} \mathrm{O}_{2}$ is a powerful oxidant as it reacts with carbon dioxides present in the air.
$\mathrm{Na}_{2} \mathrm{O}_{2}+\mathrm{CO} \rightarrow \mathrm{Na}_{2} \mathrm{CO}_{3}$
$\mathrm{Na}_{2} \mathrm{O}_{2}+2 \mathrm{CO}_{2} \rightarrow \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{O}_{2}$
$\mathrm{Na}_{2} \mathrm{O}_{2}+\mathrm{Cr}^{3+} \rightarrow \mathrm{CrO}_{4}^{2-}$
Structure
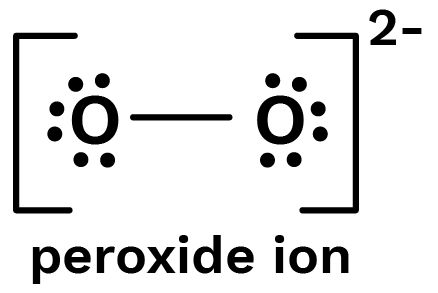
Oxygen atom is $\mathrm{sp}^{3}$ hybridized. Peroxide ion has 18 electrons which occupies the molecular orbital as shown:
$\sigma 1 \mathrm{~s}^{2}, \sigma^{*} 1 \mathrm{~s}^{2}, \sigma 2 \mathrm{~s}^{2}, \sigma^{*} 2 \mathrm{~s}^{2}, \sigma 2 \mathrm{p}_{z}^{2}, \pi 2 \mathrm{p}_{\mathrm{y}}^{2}=\pi 2 \mathrm{p}_{\mathrm{z}}^{2}, \pi^{*} 2 \mathrm{p}_{\mathrm{y}}^{2}=\pi^{*} 2 \mathrm{P}_{z}^{1}$
The bond order being 1 so, it is diamagnetic.
Superoxides
Superoxides are ionic oxides $\mathrm{M}^{+} \mathrm{O}_{2}^{-}$
Preparation
$\mathrm{M}+\mathrm{O}_{2}($ excess $) \rightarrow \mathrm{MO}_{2}$ $(\mathrm{M}=\mathrm{K}, \mathrm{Rb}, \mathrm{Cs})$
Superoxides are stronger oxidizing agents than peroxides. The stability of these superoxides is in the order:
$\mathrm{KO}_{2}<\mathrm{RbO}_{2}<\mathrm{CsO}_{2}$
Reactions
$\mathrm{KO}_{2}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{KOH}+\mathrm{H}_{2} \mathrm{O}_{2}+1 / 2 \mathrm{O}_{2}$
Because it creates $\mathrm{O}_{2}$ and eliminates $\mathrm{CO}_{2}$, $\mathrm{KO}_{2}$ is utilised in space capsules, submarines, and breathing masks.
$4 \mathrm{KO}_{2}+2 \mathrm{CO}_{2} \rightarrow 2 \mathrm{~K}_{2} \mathrm{CO}_{3}+3 \mathrm{O}_{2}$
$4 \mathrm{KO}_{2}+4 \mathrm{CO}_{2}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow 4 \mathrm{KHCO}_{3}+\mathrm{O}_{2}$
Sodium superoxide can be made by reacting sodium peroxide with oxygen at high temperatures and pressures, rather than by burning metal in oxygen.
$\mathrm{Na}_{2} \mathrm{O}+\mathrm{O}_{2} \rightarrow 2 \mathrm{NaO}_{2}$
Structure
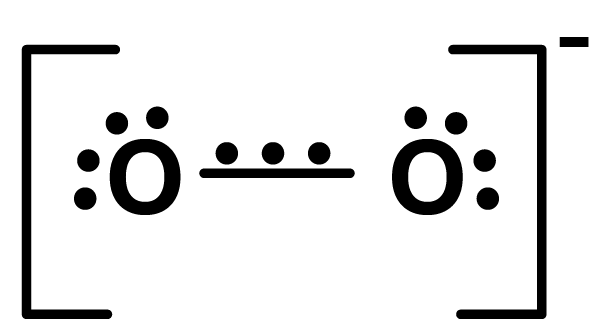
The paramagnetic property is explained by the existence of one unpaired electron in a three-electron bond. The superoxide molecule has 17 electrons and a bond order of1.5, occupying the molecular orbitals as illustrated.
$\sigma 1 \mathrm{~s}^{2}, \sigma^{*} 1 \mathrm{~s}^{2}, \sigma 2 \mathrm{~s}^{2}, \sigma^{*} 2 \mathrm{~s}^{2}, \sigma 2 \mathrm{p}_{\mathrm{x}}^{2}, \pi 2 \mathrm{p}_{\mathrm{y}}^{2}=\pi 2 \mathrm{p}_{\mathrm{z}}^{2}, \pi^{*} 2 \mathrm{p}_{\mathrm{y}}^{2}=\pi^{*} 2 \mathrm{P}_{\mathrm{z}}^{1}$
The stability of oxides is given as:
Normal oxide > peroxide > superoxide
Carbonates and Bicarbonates
Solid bicarbonates are formed by Group 1 metals $\left(\mathrm{MHCO}_{3}\right)$. $\mathrm{M}_{2} \mathrm{CO}_{3}$ carbonates are formed by all alkali metals. The carbonates and bicarbonates of alkali metals are extremely heat stable due to their electro positive character \[\mathbf{Li}_{2}\mathbf{CO}_{3}\] decomposes easily by heat).
The unusual behaviour of $\mathrm{Li}_{2} \mathrm{CO}_{3}$ can be explained by the fact that:
Lithium's small size and high polarisation disrupts the electron cloud of the nearby oxygen atom of the massive $\mathrm{CO}_{3}^{2}$, weakening the carbon-hydrogen bond.
$\mathrm{Li}_{2} \mathrm{CO}_{3} \longrightarrow \mathrm{Li}_{2} \mathrm{O}+\mathrm{CO}_{2}$
When a larger carbonate ion is replaced by a smaller carbonate ion, the lattice energy increases, favouring breakdown.
$\mathrm{M}_{2} \mathrm{CO}_{3} \longrightarrow \mathrm{M}_{2} \mathrm{O}+\mathrm{CO}_{2} \uparrow$
As a washing soda, $\mathrm{Na}_{2} \mathbf{C O}_{3}$is utilised. Baking soda is made from $\mathrm{NaHCO}_{3}$ . Both $\mathrm{NaHCO}_{3}$ and $\mathrm{KHCO}_{3}$ have hydrogen bonds in their crystal structures. The $\mathrm{HCO}_{3}^{-}$ in $\mathrm{NaHCO}_{3}$ forms an endless chain, whereas $\mathrm{KHCO}_{3}$ forms a dimeric anion.
Reactions
$2 \mathrm{HNO}_{3}+\mathrm{K}_{2} \mathrm{CO}_{3} \rightarrow 2 \mathrm{KNO}_{3}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O}$
$2 \mathrm{NaHCO}_{3} \longrightarrow \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}$
$\mathrm{M}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O} \rightleftharpoons 2 \mathrm{M}^{+}+\mathrm{HCO}_{3}^{-}+\mathrm{OH}^{-}$
Halides
All of the metals in this group create MX halides. Because lithium ion is the smallest ion in the group, it is more likely than other metals to produce hydrated salts.
Properties
Alkali metal halides are excellent ionic compounds, as evidenced by the following features.
With the exception of lithium fluoride, all alkali halides are easily soluble in water (Lithium fluoride is soluble in non-polar solvents).
Their melting and boiling points are extremely high.
The melting and boiling points of the same alkali metal drop in a predictable order.
Fluoride > chloride > bromide > iodide
This is described in terms of the metal halides' lattice energy*. The lattice energy of the same metal reduces when the halogen's electronegativity lowers.
The melting point of lithium halides is lower than that of sodium halides for the identical halide ion. However, as we travel down the group from sodium to cesium, the melting points of halides decline. Lithium halides exhibit aberrant behaviour due to their covalent character, whereas sodium and other halides are ionic in nature. As we advance along the group of ionic halides, the melting point lowers as the lattice energy decreases.
$NaCl > KCl > RbCl > CsCl$
Solubility of halides of alkali metals: Alkali metal halides have a range of solubilities. The solubility of alkali metal fluorides in water, for example, gradually increases from lithium to caesium. Lithium chloride has a far better solubility in water than sodium chloride when it comes to chlorides. This is owing to the lithium ion's tiny size and high hydration energy. However, when the lattice energy of the crystals decreases, solubility in water increases steadily from sodium chloride to cesium chloride.
In the fused condition, they are good conductors of electricity.
They are made up of ionic crystals. Lithium halides, on the other hand, have a partially covalent character due to the polarising power of lithium ions.
The lattice energy and polarising power are responsible for the structure and stability (solubility) of alkali metal halides.
Lattice Energy: The energy produced during the production of a crystal lattice from gaseous cations and anions, or the energy necessary to split one mole of a solid ionic compound into its gaseous ions, is known as lattice energy. As a result, lattice energy (the force of attraction between ions) is a direct measure of ionic crystal stability; the higher the lattice energy of a molecule, the lower its solubility in water.
When an ionic compound crystal comes into contact with a polar solvent like water, the water molecule's hydrogen end (positive pole) is attracted to a negative ion, whereas the oxygen end (negative pole) is drawn to a positive ion. Solvation (or hydration, if the solvent is water) of the ions is the process of polar solvent molecules attaching to the ions. When ions are stabilised by solvation, a considerable amount of solvation energy (or hydration energy) is released, which, if it exceeds the crystal's lattice energy, causes the ionic compound to dissolve in the solvent. In the case of lithium fluoride, however, if the solvation energy is insufficient to oppose the lattice energy, the material stays insoluble. The combination of small lithium ions and small fluoride ions gives lithium fluoride its high lattice energy. The lattice energy of a particular ion increases as the size of the oppositely charged ion decreases.
Polarising power and polarisability (Fajan’s Rule): Although an ionic bond in a chemical like $\mathrm{M}^{+} \mathrm{X}^{-}$ is thought to be 100 percent ionic, it is discovered to have significant covalent character in some circumstances (e.g., lithium halides). When two oppositely charged ions approach each other, the nature of the link between them, according to Fajan, is determined by the action of one ion on the other.
When two oppositely charged ions come into contact, the positive ion attracts electrons from the anion's outermost shell while repelling the anion's positively charged nucleus. The anion is distorted, deformed, or polarised as a result of this. The ability of a cation to distort an anion is called polarisation power, and the anion's susceptibility to be polarised by the cation is called polarisability. If the degree of polarisation is modest, an ionic bond is established; however, if the degree of polarisation is considerable, electrons are attracted from the anion to the cation by electrostatic attraction, resulting in a higher electron density between the two ions and a covalent connection. In general, the stronger an ion's polarisation power or polarisability, the greater its proclivity to form covalent bonds. Because polarisation power grows as the size of the cation decreases and polarisability increases as the size of the anion increases, the polarisation in a compound containing big negative ions and small positive ions may be so strong that the connection becomes covalent. In nature, lithium iodide, which is made up of lithium ions (the smallest alkali metal ion) and iodide ions (the largest halide ion), is found to be highly covalent.
Other examples of such ionic-covalent compounds are $\mathrm{AlCl}_{3}, \mathrm{FeCl}_{3}, \mathrm{SnCl}_{4}$, etc.
Reactions
Ionic polyhalides are formed when alkali metal halides react with halogen and interhalogen compounds.
$\mathrm{KI}+\mathrm{I}_{2} \rightarrow \mathrm{K}\left[\mathrm{I}_{3}\right]$
$\mathrm{KBr}+\mathrm{ICl} \rightarrow \mathrm{K}[\mathrm{Br} \mathrm{ICl}]$
$\mathrm{KF}+\mathrm{BrF}_{3} \rightarrow \mathrm{K}\left[\mathrm{BrF}_{4}\right]$
Sulphates
They form sulphates of type $\mathrm{M}_{2} \mathrm{SO}_{4}$
Anomalous Behaviour of Lithium
Although lithium has many of the properties of the group I elements, it also differs from them in a number of ways. The incredibly small size of the lithium atom and its ion causes this unusual behaviour. The high charge density of the lithium ion is due to its small size. As a result, out of all the alkali metal ions, lithium ion has the most polarising power. As a result, it has a significant distorting impact on a negative ion. As a result, the lithium ion has a strong proclivity for solvation and the creation of covalent bonds. It's also worth noting that the polarising power of the lithium ion is similar to that of the magnesium ion, making the two elements resemble very much in their properties.
Lithium is substantially more difficult to work with than the other elements in Group I. (similarity with magnesium which is also a hard metal).
Lithium has a high melting point and boiling point.
Lithium, unlike the other elements in this group, is the least reactive, as shown by the following points.
It is not influenced by air, unlike others.
It, unlike others, decomposes water slowly (similar to magnesium).
It rarely reacts with bromine, unlike many others.
On burning in oxygen, it forms only the monoxide $\mathrm{Li}_{2} \mathrm{O}$ , while the others form peroxides too.
Unlike other elements, it forms nitride when it comes into contact with nitrogen, $\mathrm{Li}_{3} \mathrm{~N}$ (similarity with $\mathrm{Mg}$ )
Lithium is much less electropositive and, therefore, several of its compounds $\left(\mathrm{Li}_{2} \mathrm{CO}_{3}\right.$ and $\left.\mathrm{LiOH}\right)$ are less stable (similarity with Mg). For example,
$2 \mathrm{LiOH} \rightarrow \mathrm{Li}_{2} \mathrm{O}+\mathrm{H}_{2} \mathrm{O}$
$\mathrm{Mg}(\mathrm{OH})_{2} \rightarrow \mathrm{MgO}+\mathrm{H}_{2} \mathrm{O}$
When heated, lithium nitrate produces nitrogen dioxide and oxygen, leaving lithium oxide behind (similar to magnesium nitrate), but sodium and potassium nitrates produce only oxygen, leaving nitrites.
The majority of lithium salts (for example, hydroxide, carbonate, oxalate, phosphate, and fluoride) are water insoluble (similarity with magnesium.) The sodium and potassium salts that correspond to them are both water soluble.
Lithium halides and lithium alkyls are soluble in organic solvents, but sodium and potassium halides and alkyls are not; $ \mathrm{MgCl}_{2}$ is soluble in alcohol as well.
Lithium chloride, like magnesium chloride, undergoes some hydrolysis in hot water, but only to a little level; sodium chloride and potassium chloride do not.
Lithium sulphate, unlike sulphates of other alkali metals, does not create alums.
Partially covalent lithium compounds, particularly lithium halides, exist in nature. This is owing to lithium ions' proclivity for attracting electrons (polarising power). This explains why lithium compounds have a smaller dipole moment than expected.
The ions and compounds of alkali metals are more hydrated than those of other alkali metals (similarity with magnesium).
Extraction of Sodium
Sodium is obtained on large scale by two process:
Castner’s process
The electrolysis of fused sodium hydroxide takes place at $330^{\circ} \mathrm{C}$ , with iron as the cathode and nickel as the anode.
$2 \mathrm{NaOH} \rightleftharpoons 2 \mathrm{Na}^{+}+2 \mathrm{OH}^{-}$
At cathode: \[2 \mathrm{Na}^{+}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Na}\]
At anode: $4 \mathrm{OH}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O}+\mathrm{O}_{2}+4 \mathrm{e}^{-}$
Oxygen and water are created during electrolysis. At the cathode, water generated at the anode is partially evaporated and partially broken down, resulting in hydrogen discharge.
$\mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{H}^{+}+\mathrm{OH}^{-}$
At cathode: \[2\mathbf{H}^{+}+2 \mathbf{e} \rightarrow 2 \mathbf{H} \rightarrow \mathbf{H}_{2}\uparrow\]
Down’s Process
Nowadays, the metal is produced using Down's technique. It includes employing iron as a cathode and graphite as an anode to electrolyze fused sodium chloride containing calcium chloride and potassium fluoride at roughly 600 degrees Celsius. The cell is made up of a steel tank with heat-resistant bricks lining it. In the centre of the cell, a circular graphite anode is installed, which is encircled by a cylindrical iron cathode. A steel gauze cylinder separates the anode and cathode, allowing fused charge to pass through. A dome-shaped steel hood protects the anode and provides an outlet for chlorine gas to escape. The molten metal that has been freed at the cathode rises and flows into the kerosene receiver.
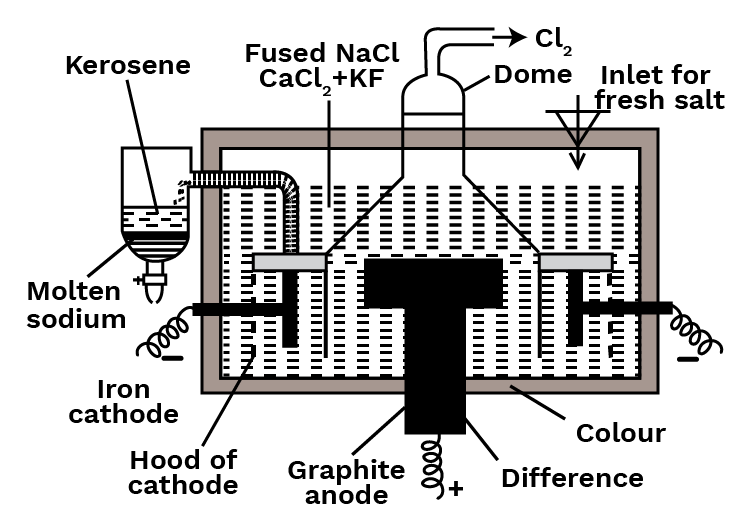
Reactions: $\mathrm{NaCl} \rightleftharpoons \mathrm{Na}^{+}+\mathrm{Cl}^{-}$
At Cathode: $2 \mathrm{Na}^{+}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Na}$
At Anode: $2 \mathrm{Cl}^{-} \rightarrow \mathrm{Cl}_{2}+2 \mathrm{e}^{-}$
The sodium chloride when obtained from this method is $99.5 \%$ pure.
The electrolysis of pure sodium chloride has a number of drawbacks:
Sodium chloride has a high fusion temperature of 803 degree celsius, which is difficult to sustain.
Because sodium is volatile at this temperature, some of it vapourizes, forming a metallic fog.
The electrolysis products, salt and chlorine, are corrosive at this temperature and may harm the cell's substance.
Pure sodium chloride is blended with calcium chloride and potassium fluoride to overcome the problems mentioned above. At the voltage used, calcium chloride and potassium fluoride do not breakdown, but they do lower the fusion temperature. A combination containing 40% sodium chloride, 60% calcium chloride, and a trace amount of potassium fluoride reaches a fusion temperature of around 600 degrees Celsius. In the electrolytic cell, this combination is electrolyzed at 600 degrees Celsius.
Example 1: Alkali metals are paramagnetic but their salts are diamagnetic. Explain.
Solution: The outermost energy shell in metals is solely occupied, whereas in cations, all orbitals are double occupied (inert gas configuration).
e.g., $\quad \mathrm{Na}, 1 \mathrm{~s}^{2}, 2 \mathrm{~s}^{2} 2 \mathrm{p}^{6}, 3 \mathrm{~s}^{2} 3 \mathrm{p}^{6}$, 4s $^{1}$ paramagnetic
$\mathrm{Na}^{+} 1 \mathrm{~s}^{2}, 2 \mathrm{~s}^{2} 2 \mathrm{p}^{6},\left(3 \mathrm{~s}^{2} 3 \mathrm{p}^{6}\right)$ Diamagnetic
Example 2: Alkali metals are good reducing agents. Explain.
Solution: Alkali metals are strong reducing agents because their low ionisation enthalpy values and high oxidation potential allow them to easily lose valence electrons.
Example 3: Which alkali metal ion has the maximum polarising power and why?
Solution: Among the alkali metal ions, the lithium ion has the most polarising power. This is owing to the lithium ion's small size.
Example 4: Lithium ion is far smaller than other alkali metal ions but it moves through a solution less rapidly that the others. Explain.
OR
The conductance of lithium salts is less in comparison to the salts of other alkali metals. Explain.
Solution: Lithium ion has the highest degree of hydration because of its strong charge, which pulls numerous water molecules surrounding it. As a result, the size of the hydrated lithium ion is larger than that of the other alkali metal ions, affecting its mobility in solution and lowering conductance.
Size: $\quad[\mathrm{Li}(\mathrm{aq})]^{+}>[\mathrm{Na}(\mathrm{aq})]^{+}>[\mathrm{K}(\mathrm{aq})]^{+}$
Example 5: Sodium salts in aqueous solutions are either neutral or alkaline in nature. Explain.
Solution: Strong acids or weak acids produce the anions in sodium salts. There is no hydrolysis when anions come from strong acids, and aqueous solutions are neutral. When anions come from weak acids, however, they undergo hydrolysis, resulting in alkaline solutions. Solns. of sodium carbonate or bicarbonate, for example, are alkaline.
\[\mathrm{CO}_{3}^{2-}+\mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{HCO}_{3}^{-}+\mathrm{OH}^{-}\]
$\mathrm{HCO}_{3}^{-}+\mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{H}_{2} \mathrm{CO}_{3}+\mathrm{OH}^{-}$
Example 6: Why do potassium, rubidium and cesium form superoxides in preference to oxides and peroxides on being heated in excess supply of air?
Solution: $\mathrm{K}^{+}, \mathrm{Rb}^{+}$and $\mathrm{Cs}^{+}$are large cations in size and superoxide ion $\left(\mathrm{O}_{2}^{-}\right)$is larger in size in comparison to oxide $\left(\mathrm{O}^{2-}\right)$ and peroxide $\left(\mathrm{O}_{2}^{2-}\right)$ ion. These metals form superoxides rather than oxides or peroxides because a larger cation can stabilise a large anion.
Example 7: Why is ${\text{K}}{{\text{O}}_{\text{2}}}$ paramagnetic?
Solution: The superoxide $\mathrm{O}_{2}^{-}$is paramagnetic because of one unpaired electron in $\pi * 2 \mathrm{p}$ molecular orbital.
$\mathrm{KK} \sigma(2 \mathrm{~s})^{2} \sigma^{*}(2 \mathrm{~s})^{2} \sigma\left(2 \mathrm{p}_{\mathrm{x}}\right)^{2} \pi\left(2 \mathrm{p}_{\mathrm{x}}\right)^{2}\left(\pi 2 \mathrm{p}_{\mathrm{y}}\right)^{2} \pi^{*}\left(2 \mathrm{p}_{\mathrm{x}}\right)^{2}$ $\pi^{*}\left(2 \mathrm{p}_{\mathrm{v}}\right)^{1}$
Example 8: Among the alkali metals which element has
Highest melting point
Highest size of hydrated ion in solution
Strongest reducing agent in solution
Least electronegative
Solution: The elements which have:
Highest melting point: Lithium
Highest size of hydrated ion in solution: $[\mathrm{Li}(\mathrm{aq})]^{+}$
Strongest reducing agent in solution: Lithium
Least electronegative: Cesium
Example 9: What happens when following compounds are heated?
$L{i_2}C{O_3} \to L{i_2}O + C{O_2}$
$N{a_2}C{O_3}.10{H_2}O \to N{a_2}C{O_3} + 10{H_2}O$
$4LiN{O_3} \to 2L{i_2}O + 4N{O_2} + {O_2}$
$2NaN{O_3} \to 2NaN{O_2} + {O_2}$
Example 10:
Arrange $\mathrm{LiF}, \mathrm{NaF}, \mathrm{KF}, \mathrm{RbF}$ and CsF in order of increasing lattice energy.
Arrange the following in order of the increasing covalent character. $\mathrm{MCl}, \mathrm{MBr}, \mathrm{MF}, \mathrm{MI}$ (where $\mathrm{M}=$ alkali metal)
Solution:
$\mathrm{CsF}<\mathrm{RbF}<\mathrm{KF}<\mathrm{NaF}<\mathrm{LiF}$
$\mathrm{MF}<\mathrm{MCl}<\mathrm{MBr}<\mathrm{MI}$
With increasing size of the anion, covalent character increases.
Example 11: Why a standard solution of sodium hydroxide cannot be prepared by weighing ?
Solution: The material sodium hydroxide is deliquescent. It absorbs moisture and reacts with atmospheric carbon dioxide, both of which increase its mass. As a result, precise weighing is difficult.
Example 12: What happens when:
Fused sodium reacts with dry ammonia.
Sodium hydrogen carbonate is heated.
Sodium hydroxide is heated with sulphur?
Solution:
$2 \mathrm{Na}+2 \mathrm{NH}_{3}+\rightarrow 2 \mathrm{NaNH}_{2}+\mathrm{H}_{2}$
Sodium carbonate is formed. $2 \mathrm{NaHCO}_{3} \rightarrow \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}$
Sodium thiosulphate is formed.
$4 \mathrm{~s}+6 \mathrm{NaOH} \rightarrow \mathrm{Na}_{2} \mathrm{~S}_{2} \mathrm{O}_{3}+2 \mathrm{Na}_{2} \mathrm{~S}+3 \mathrm{H}_{2} \mathrm{O}$
Example 13: Give reasons for the following:
$\mathrm{LiCl}$ is more covalent than $\mathrm{NaCl}$
Lithium Iodide has lower melting point then $\mathrm{LiCl}$
$\mathrm{MgCl}_{2}$ is more covalent than $\mathrm{NaCl}$
$\mathrm{CuCl}$ is more covalent than $\mathrm{NaCl}$
Solution:
Lithium ion is more polarising than sodium ion due to its smaller size, and so lithium chloride is more covalent than sodium chloride.
Due to bigger size, $\mathrm{I}^{-}$ is more polarisable than $\mathrm{Cl}^{-}$ and hence lithium iodide is more covalent than lithium chloride. Therefore, lithium iodide has lower melting point than $\mathrm{LiCl}$.
Magnesium ion is more polarising than sodium ion due to its greater charge, and so magnesium chloride is more covalent than sodium chloride.
Copper ion is more polarising than sodium ion because to the pseudo inert gas configuration, and so copper chloride is more covalent than sodium chloride.
Example 14: Identify (A), (B), (C) and (D) and give their chemical formulae.
(A) $+\mathrm{NaOH} \quad$ Heat ${\longrightarrow} \mathrm{NaCl}+\mathrm{NH}_{3}+\mathrm{H}_{2} \mathrm{O}$
$\mathrm{NH}_{3}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O} \longrightarrow(\mathrm{B})$
$(\mathrm{B})+\mathrm{NaCl} \longrightarrow(\mathrm{C})+\mathrm{NH}_{4} \mathrm{Cl}$
(C) Heat $\mathrm{Na}_{2} \mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O}+(\mathrm{D})$
Solution:
$\mathrm{NH}_{4} \mathrm{Cl}+\mathrm{NaOH}_{\longrightarrow}{\text { Heat }}{\longrightarrow} \mathrm{NH}_{3}+\mathrm{NaCl}+\mathrm{H}_{2} \mathrm{O}$
Compound A is ammonium chloride $\left(\mathrm{NH}_{4} \mathrm{Cl}\right)$.
$\mathrm{NH}_{3}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O} \longrightarrow \mathrm{NH}_{4} \mathrm{HCO}_{3}$
Compound B is ammonium bicarbonate $\left(\mathrm{NH}_{4} \mathrm{HCO}_{3}\right)$.
$\mathrm{NH}_{4} \mathrm{HCO}_{3}+\mathrm{NaCl} \longrightarrow \mathrm{NaHCO}_{3}+\mathrm{NH}_{4} \mathrm{Cl}$
Compound (C) is sodium bicarbonate $\left(\mathrm{NaHCO}_{3}\right)$.
$2 \mathrm{NaHCO}_{3} \longrightarrow \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}$
Compound (D) is carbon dioxide $\left(\mathrm{CO}_{2}\right)$.
Example 15: Arrange the following as specified:
$\mathrm{MgO}, \mathrm{SrO}, \mathrm{K}_{2} \mathrm{O}$ and $\mathrm{Cs}_{2} \mathrm{O}$ (increasing order of basic character)
$\mathrm{LiCl}$, ${\text{LiBr}}$ , ${\text{LiI}}$ (decreasing order of covalent character)
$\mathrm{NaHCO}_{3}, \mathrm{KHCO}_{3}, \mathrm{Mg}\left(\mathrm{HCO}_{3}\right)_{2}, \mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}$ (decreasing solubility in water)
$\mathrm{LiF}, \mathrm{NaF}, \mathrm{RbF}, \mathrm{KF}$ and CsF (in order of increasing lattice energy)
$\mathrm{Li}, \mathrm{Na} \mathrm{K}$ (in order to decreasing reducing nature in solution)
Solution:
$\mathrm{MgO}<\mathrm{SrO}<\mathrm{K}_{2} \mathrm{O}<\mathrm{Cs}_{2} \mathrm{O}$}
$\mathrm{LiI}>\mathrm{LiBr}>\mathrm{LiCl}$
$\mathrm{NaHCO}_{3}<\mathrm{KHCO}_{3}<\mathrm{Mg}\left(\mathrm{HCO}_{3}\right)_{2}<\mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}$
$\mathrm{CsF}<\mathrm{RbF}<\mathrm{KF}<\mathrm{NaF}<\mathrm{LiF}$
$\mathrm{Li}>\mathrm{K}>\mathrm{Na}$
Example 16:
What happens when $\mathrm{KO}_{2}$ reacts with water ? Give the balanced chemical equation.
Predict giving reason the outcome of the reaction :
$\mathrm{LiI}+\mathrm{KF} \longrightarrow$
Solution:
When $\mathrm{KO}_{2}$ reacts with water, oxygen is evolved and an alkaline solution containing potassium hydroxide and $\mathrm{H}_{2} \mathrm{O}_{2}$ is formed:
$2\mathrm{KO}_{2}+2 \mathrm{H}_{2} \mathrm{O} \longrightarrow 2 \mathrm{KOH}+\mathrm{H}_{2} \mathrm{O}_{2}+\mathrm{O}_{2}$
Lithium iodide reacts with potassium fluoride and anions are exchanged in this process:
$\mathrm{LiI}+\mathrm{KF} \longrightarrow \mathrm{LiF}+\mathrm{KI}$
As stable compounds form, the exchange takes place, with the bigger cation stabilising the larger anion and the smaller cation stabilising the smaller anion.
Alkaline Earth Metals
Introduction
Group-II of the periodic table contains the elements beryllium, magnesium, calcium, strontium, barium, and radium.
All of these substances are metals. Calcium, strontium, and barium oxides were discovered far before the metals themselves, and they were dubbed alkaline earths because they were alkaline and found in the earth. Alkaline earth metals were given to the elements once they were found. Radium shares chemical properties with alkaline earth metals, but because it is a radioactive element, it is researched independently from the other radioactive elements.
Physical Properties
Atomic Size
On going down the group, atomic size of elements increases.
Oxidation State
+2 oxidation state is exhibited by group II elements.
Density
Group II elements are smaller in size than group I elements, hence they have a higher density than group I elements. From beryllium to radon, density rises.
Exception: Calcium has a lower density than magnesium, whereas magnesium has a lower density than beryllium.
Nature of Bonds
Beryllium forms mainly covalent compound. The rest of the elements in group II form ionic bonds.
Hydration Energy
Because of their smaller size and higher charge, the hydration energies of group 2 ions are four to five times higher than those of group 1 ions. As the size of the ions grows larger, the hydration enthalpy falls.
Lattice Energy
The lattice energy of alkali metal salts with a common anion drops as one moves down the group.
Ionization Energy
Because the atoms in group 2 are smaller, the electrons are more firmly bound, requiring more energy to remove the initial electron (first ionisation energy) than in group 1. The amount of energy necessary to remove the second electron is approximately double that required to remove the first. As a result, the energy necessary to make divalent ions from group 2 elements is four times that required to make $\mathrm{M}^{+}$ from group 1 metals.
Flame Test
These elements' electrons are stimulated to higher energy levels when energy is delivered to them in a flame, as is the case with alkali metals under identical conditions. The excess energy generated by the electrons when they return to their original energy level produces visible light with distinct colours, as shown below:
Element | Colour |
Calcium | Brick red |
Strontium | Crimson red |
Barium | Grassy green |
Radium | Crimson |
The atoms of beryllium and magnesium are smaller. As a result, the electrons in these atoms are more tightly bound. As a result, the energy of the flame does not stimulate them to higher energy levels. As a result, these ingredients do not produce any colour in the bunsen flame.
Standard Oxidation Potential
Standard Oxidation Potential of Alkaline Earth Metals
Element | Oxidation Reaction | Standard Oxidation Potential (volt) |
$\mathrm{Be}$ | $\mathrm{Be} \rightarrow \mathrm{Be}^{2+}+2 \mathrm{e}^{-}$ | $1.85$ |
$\mathrm{Mg}$ | $\mathrm{Mg} \rightarrow \mathrm{Mg}^{2+}+2 \mathrm{e}^{-}$ | $2.37$ |
$\mathrm{Ca}$ | $\mathrm{Ca} \rightarrow \mathrm{Ca}^{2+}+2 \mathrm{e}^{-}$ | $2.87$ |
$\mathrm{Sr}$ | $\mathrm{Sr} \rightarrow \mathrm{Sr}^{2+}+2 \mathrm{e}^{-}$ | $2.89$ |
$\mathrm{Ba}$ | $\mathrm{Ba} \rightarrow \mathrm{Ba}^{2+}+2 \mathrm{e}^{-}$ | $2.90$ |
Solubility in Liquid Ammonia
The metals, like group 1 metals, dissolve in liquid ammonia. Due to the creation of solvated electrons, dilute solutions are blue in colour. As the solution decomposes, amides form and hydrogen gas is released.
$2 \mathrm{NH}_{3}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{NH}_{2}^{-}+\mathrm{H}_{2}$
Electronegative Values
Group II element’s electronegativity values are low, although they are higher than group I element’s. The value of electronegativity diminishes as you progress through the group.
Colourless and Diamagnetism
The elements of the alkaline earth metal group generate $\mathrm{M}^{2+}$ ions, which are diamagnetic and colourless due to the lack of an unpaired electron.
Melting and Boiling Point
The melting point of elements in group II lowers as the cohesive force diminishes as the group progresses.
Exception: Magnesium has the lowest melting point
Metallic Properties
Group-II elements have typical metallic characteristics. They have a nice metallic sheen and excellent electrical and thermal conductivity.
GROUP – I and II
Oxides
Sodium Oxide
Preparation
It's made by heating sodium to 180 degrees Celsius in a small amount of air or oxygen and then distilling the surplus sodium away.
$2 \mathrm{Na}+1 / 2 \mathrm{O}_{2} \stackrel{180^{\circ}}{\longrightarrow} \mathrm{Na}_{2} \mathrm{O}$
By heating sodium peroxide, nitrate or nitrate with sodium.
$N{a_2}{O_2} + 2Na \to 2N{a_2}O$
$2NaN{O_3} + 10Na \to 6N{a_2}O + {N_2}$
$2NaN{O_2} + 6Na \to 4N{a_2}O + {N_2}$
Properties
It is a white amorphous mass.
It gets decomposed at 400 degree Celsius into sodium peroxide and sodium.
$2 \mathrm{Na}_{2} \mathrm{O} \stackrel{400^{\circ} \mathrm{C}}{\longrightarrow} \mathrm{Na}_{2} \mathrm{O}_{2}+2 \mathrm{Na}$
It gets dissolved violently in water and yields caustic soda.
$\mathrm{Na}_{2} \mathrm{O}+\mathrm{H}_{2} \mathrm{O} \longrightarrow 2 \mathrm{NaOH}$
Sodium Peroxides
Preparation:
It is formed by heating the metal in excess of air or oxygen at 300 degrees Celsius in a dry, carbon dioxide-free environment.
$2 \mathrm{Na}+\mathrm{O}_{2} \longrightarrow \mathrm{Na}_{2} \mathrm{O}_{2}$
Properties
It is a pale yellow solid, becoming white in air from the formation of a film of $\mathrm{NaOH}$ and $\mathrm{Na}_{2} \mathrm{CO}_{3}$ .
In cold water, sodium peroxide produces $\mathrm{H}_{2} \mathrm{O}_{2}$ but at room temperature it produces oxygen. Sodium peroxide produces hydrogen peroxide in ice-cold mineral acids.
$\mathrm{Na}_{2} \mathrm{O}_{2}+2 \mathrm{H}_{2} \mathrm{O} \stackrel{\sim 0^{\circ} \mathrm{C}}{\longrightarrow} 2 \mathrm{NaOH}+\mathrm{H}_{2} \mathrm{O}_{2}$
$2 \mathrm{Na}_{2} \mathrm{O}_{2}+2 \mathrm{H}_{2} \mathrm{O} \stackrel{25^{\circ} \mathrm{C}}{\longrightarrow} 4 \mathrm{NaOH}+\mathrm{O}_{2}$
$\mathrm{Na}_{2} \mathrm{O}_{2}+\mathrm{H}_{2} \mathrm{SO}_{4} \stackrel{\sim 0^{\circ} \mathrm{C}}{\longrightarrow} \mathrm{Na}_{2} \mathrm{SO}_{4}+\mathrm{H}_{2} \mathrm{O}_{2}$
It combines with carbon dioxide to produce sodium carbonate and oxygen, which is why it's used to purify air in small spaces. Example: submarine, ill-ventilated room.
$2 \mathrm{Na}_{2} \mathrm{O}_{2}+2 \mathrm{CO}_{2} \longrightarrow 2 \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{O}_{2}$
It is an oxidising agent and oxidises charcoal, $\mathrm{CO}, \mathrm{NH}_{3}$, $\mathrm{SO}_{2}$
$3 \mathrm{Na}_{2} \mathrm{O}_{2}+2 \mathrm{C} \longrightarrow 2 \mathrm{Na}_{2} \mathrm{CO}_{3}+2 \mathrm{Na}$
$\mathrm{Na}_{2} \mathrm{O}_{2}+\mathrm{CO} \longrightarrow \mathrm{Na}_{2} \mathrm{CO}_{3}$
$\mathrm{Na}_{2} \mathrm{O}_{2}+\mathrm{SO}_{2} \longrightarrow \mathrm{Na}_{2} \mathrm{SO}_{4}$
$3 \mathrm{Na}_{2} \mathrm{O}_{2}+2 \mathrm{NH}_{3}+\longrightarrow 6 \mathrm{NaOH}+\mathrm{N}_{2}$
It contains peroxide ion
Uses:
For preparing $\mathrm{H}_{2} \mathrm{O}_{2}, \mathrm{O}_{2}$
Oxygenating the air in submarines
Oxidising agent in the laboratory.
Oxides of Potassium:
Column | |
$\mathrm{K}_{2} \mathrm{O}$ | White |
$\mathrm{K}_{2} \mathrm{O}_{2}$ | White |
$\mathrm{K}_{2} \mathrm{O}_{3}$ | Red |
$\mathrm{KO}_{2}$ | Bright Yellow |
$\mathrm{KO}_{3}$ | Reddish Brown Needles |
Preparation:
$2KN{O_3} + 10K\xrightarrow{{heating}}6{K_2}O + {N_2}$
$\mathrm{K}_{2} \mathrm{O} \stackrel{\text { heating }}{\longrightarrow} \mathrm{K}_{2} \mathrm{O}$
${K_2}O + {H_2}O \to 2KOH$
$2 \mathrm{~K}+\mathrm{O}_{2} \stackrel{\text { Controlled }}{\text { air at } 300^{\circ} \mathrm{C}} \mathrm{K}_{2} \mathrm{O}_{2}$
Passage of $\mathrm{O}_{2}$ through a blue solution of $\mathrm{K}$ in liquid $\mathrm{NH}_{3}$ yields oxides $\mathrm{K}_{2} \mathrm{O}_{2}$ (white), $\mathrm{K}_{2} \mathrm{O}_{3}($ red $)$ and $\mathrm{KO}_{2}$ (deep yellow)
$\mathrm{K} \text { in liq. } \mathrm{NH}_{3} \longrightarrow \mathrm{K}_{2} \mathrm{O}_{2} \longrightarrow \mathrm{K}_{2} \mathrm{O}_{3} \longrightarrow \mathrm{KO}_{2}$
$2 \mathrm{KO}_{2}+2 \mathrm{H}_{2} \mathrm{O} \longrightarrow 2 \mathrm{KOH}+\mathrm{H}_{2} \mathrm{O}_{2}+\mathrm{O}_{2}$
Magnesium Oxide:
It's also known as magnesia, and it's made from natural magnesite that's been heated.
$\mathrm{MgCO}_{3} \longrightarrow \mathrm{MgO}+\mathrm{CO}_{2}$
Properties
It is present as a white powder
The melting point of magnesium oxide is 2850 degree Celsius. So, it is used in the manufacturing of refractory bricks for furnances.
It imparts alkaline reaction and it is very slightly soluble in water.
Calcium Oxide
It is manufactured by dissolving lime stone at a high temperature of roughly 1000 degrees Celsius and is known as fast lime or lime.
$CaC{O_3} \to CaO + C{O_2} + 4200cal$
Properties
It is a white amorphous powder having a melting point of 2570 degree Celsius.
When heated in an oxygen-hydrogen flame, it produces strong light (lime light).
It is a basic oxide that reacts with acidic oxides, such as sulphur dioxide.
$\mathrm{CaO}+\mathrm{SiO}_{2} \longrightarrow \mathrm{CaSiO}_{3}$
$\mathrm{CaO}+\mathrm{CO}_{2} \longrightarrow \mathrm{CaCO}_{3}$
On combination with water, it produces slaked lime
$\mathrm{CaO}+\mathrm{H}_{2} \mathrm{O} \longrightarrow \mathrm{Ca}(\mathrm{OH})_{2}$
Magnesium Peroxide and Calcium Peroxide:
These are obtained by passing $\mathrm{H}_{2} \mathrm{O}_{2}$ in a suspension of $\mathrm{Mg}(\mathrm{OH})_{2}$ and $\mathrm{Ca}(\mathrm{OH})_{2}$
Uses:
Magnesium peroxide is a whitening agent and an antimicrobial in tooth paste.
Hydroxides
Sodium Hydroxides
Preparation
Electrolysis of Brine:
$\mathrm{NaCl} \rightleftharpoons \mathrm{Na}^{+}+\mathrm{Cl}^{-}$
At Anode: $2 \mathrm{Cl}^{-} \longrightarrow \mathrm{Cl}_{2}+2 \mathrm{e}$
At Cathode: $ \mathrm{Na}+\mathrm{e}^{-} \longrightarrow \mathrm{Na}$
$2 \mathrm{Na}+2 \mathrm{H}_{2} \mathrm{O} \longrightarrow 2 \mathrm{NaOH}+\mathrm{H}_{2}$
Caustication of $\mathrm{Na}_{2} \mathrm{CO}_{3}$ (Gossage's method):
$\mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{Ca}(\mathrm{OH})_{2} \rightleftharpoons 2 \mathrm{NaOH}+\mathrm{CaCO}_{3}$
Since the $\mathrm{K}_{\mathrm{sp}}\left(\mathrm{CaCO}_{3}\right)<\mathrm{K}_{\mathrm{sp}}\left(\mathrm{Ca}(\mathrm{OH})_{2}\right)$, the reaction shifts towards right.
Properties
It's a crystalline white solid that's highly corrosive and deliquescent.
It is resistant to heat.
Its aqueous solution has an alkaline pH and feels soapy to the touch.
$\mathrm{FeCl}{3}+3 \mathrm{NaOH} \longrightarrow \mathrm{Fe}(\mathrm{OH}){3} \downarrow+3 \mathrm{NaCl}$
$\mathrm{NH}{4} \mathrm{Cl}+\mathrm{NaOH}{ } \longrightarrow \mathrm{NaCl}+\mathrm{NH}{3} \uparrow+\mathrm{H}{2} \mathrm{O}$
$\mathrm{ZnCl}_{2}+2 \mathrm{NaOH} \longrightarrow Z n(\mathrm{OH}), \downarrow+2 \mathrm{NaCl}$
$\mathrm{Zn}(\mathrm{OH}), \downarrow+2 \mathrm{NaOH}{\text {Excess }}{\longrightarrow} \mathrm{Na}{1} \mathrm{ZnO}_{2}+2 \mathrm{H}, \mathrm{O}$
Acidic and amphoteric oxidise gets dissolved easily, e.g:
$\mathrm{CO}_{2}+2 \mathrm{NaOH} \longrightarrow \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}$
$\mathrm{Al}_{2} \mathrm{O}_{3}+2 \mathrm{NaOH} \longrightarrow 2 \mathrm{NaAlO}_{2}+\mathrm{H}_{2} \mathrm{O}$
Aluminium and Zn metal gives $\mathrm{H}_{2}$ from $\mathrm{NaOH}$
$2 \mathrm{Al}+2 \mathrm{NaOH}+2 \mathrm{H}_{2} \mathrm{O} \longrightarrow 3 \mathrm{H}_{2}+2 \mathrm{NaAlO}_{2}$
Several non metals such as phosphorous, sulphur, calcium etc. yield a hydride instead of hydrogen e.g.
$4 \mathrm{P}+3 \mathrm{NaOH}+3 \mathrm{H}_{2} \mathrm{O} \longrightarrow \mathrm{PH}_{3}+3 \mathrm{NaH}_{2} \mathrm{PO}_{2}$
Potassium Hydroxide
Preparation: It is prepared by the electrolysis of aqueous solution of potassium chloride.
Properties
It is stronger base compared to sodium hydroxide
Potassium hydroxide is more soluble in water as compared to sodium hydroxide.
In alcohol, $\mathrm{NaOH}$ is sparingly soluble but $\mathrm{KOH}$ is highly soluble.
As a reagent $\mathrm{KOH}$ is less frequently used but in absorption of $\mathrm{CO}_{2}, \mathrm{KOH}$ is preferably used compared to $\mathrm{NaOH}$. Because $\mathrm{KHCO}_{3}$ formed is soluble whereas $\mathrm{NaHCO}_{3}$ is insoluble.
Magnesium Hydroxide
It is found as the mineral brucite in nature.
Preparation:
It's made by combining a caustic soda solution with a magnesium sulphate or chloride solution.
$\mathrm{MgSO}_{4}+2 \mathrm{NaOH} \longrightarrow \mathrm{Na}_{2} \mathrm{SO}_{4}+\mathrm{Mg}(\mathrm{OH})_{2}$
Properties
It can only be dried at temperatures up to 100 degrees Celsius; otherwise, at greater temperatures, it will break down into its oxide.
$\mathrm{Mg}(\mathrm{OH})_{2} \longrightarrow \mathrm{MgO}+\mathrm{H}_{2} \mathrm{O}$
It is alkalinizing because it is mildly soluble in water.
It dissolves in $\mathrm{NH}_{4} \mathrm{Cl}$ solution
$\mathrm{Mg}(\mathrm{OH})_{2}+2 \mathrm{NH}_{4} \mathrm{Cl} \longrightarrow \mathrm{MgCl}_{2}+2 \mathrm{NH}_{4} \mathrm{OH}$
Thus, $\mathrm{Mg}(\mathrm{OH})_{2}$ is not therefore precipitated from a solution of $\mathrm{Mg}^{+2}$ ions by $\mathrm{NH}_{4} \mathrm{OH}$ in presence of excess of $\mathrm{NH}_{4} \mathrm{Cl}$
Calcium Hydroxide
Preparation: It can be prepared easily by spraying water on quicklime.
$\mathrm{CaO}+\mathrm{H}_{2} \mathrm{O} \longrightarrow \mathrm{Ca}(\mathrm{OH})_{2}$
Properties
The solubility in water of calcium hydroxide is very less.
It has a lower solubility in hot water than in cold water. When a result, as the temperature rises, so does the solubility.
Carbon dioxide is readily absorbed by calcium hydroxide and is used as a test for the gas.
CARBONATES
Preparation
Leblanc Process:
$\mathrm{NaCl}+\mathrm{H}_{2} \mathrm{SO}_{4}$ (conc.) $\stackrel{\text { mild heating }}{\longrightarrow} \mathrm{NaHSO}_{4}+\mathrm{HCl}$
$\mathrm{NaCl}+\mathrm{NaHSO}_{4} \quad \stackrel{\text { Strongly }}{\text { heated }}{\longrightarrow} \mathrm{Na}_{2} \mathrm{SO}_{4}+\mathrm{HCl}$
$\mathrm{Na}_{2} \mathrm{SO}_{4}+4 \mathrm{C} \longrightarrow \mathrm{Na}_{2} \mathrm{~S}+4 \mathrm{CO} \uparrow$
$\mathrm{Na}_{2} \mathrm{~S}+\mathrm{CaCO}_{3} \longrightarrow \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{CaS}$
Solvay Process:
$\mathrm{NH}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2} \longrightarrow \mathrm{NH}_{4} \mathrm{HCO}_{3}$
$\mathrm{NaCl}+\mathrm{NH}_{4} \mathrm{HCO}_{3} \longrightarrow \mathrm{NaHCO}_{3}+\mathrm{NH}_{4} \mathrm{Cl}$
$2 \mathrm{NaHCO}_{3} \stackrel{150^{\circ} \mathrm{C}}{\longrightarrow} \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}$
Properties
Soda ash is anhydrous sodium carbonate that does not disintegrate when heated but melts at 852 degrees Celsius.
Sodium carbonate forms a number of hydrates.
Hydrated $\mathrm{Na}_{2} \mathrm{CO}_{3}$ is called washing soda $\left(\mathrm{Na}_{2} \mathrm{CO}_{3} \cdot 10\right.$ $\left.\mathrm{H}_{2} \mathrm{O}\right)$ and is prepared by Le Blanc process solvay process and electrolytic process.
Sodium carbonate absorbs carbon dioxide and produces sodium bicarbonate, which can be calcined to obtain pure sodium carbonate at 250 degrees Celsius.
$\mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2} \underset{\sum_{250^{\circ} \mathrm{C}}} 2 \mathrm{NaHCO}_{3}$
It was causticized by lime after being dissolved in acid with effervescence of carbon dioxide.
$\mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{HCl} \longrightarrow 2 \mathrm{NaCl}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}$
$\mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{Ca}(\mathrm{OH})_{2} \longrightarrow 2 \mathrm{NaOH}+\mathrm{CaCO}_{3}$
Uses: Sodium carbonate is widely used as a smelter in glass making
Potassium Carbonate
It can be made by the leblanc method, but not by the solvay process since potassium carbonate is water soluble.
Properties: It resembles with $\mathrm{Na}_{2} \mathrm{CO}_{3}$, its melting point is $900^{\circ} \mathrm{C}$ but a mixture of $\mathrm{Na}_{2} \mathrm{CO}_{3}$ and $\mathrm{K}_{2} \mathrm{CO}_{3}$ melts at $712^{\circ} \mathrm{C}$.
Uses: Potassium carbonate is used in glass manufacturing.
Calcium Carbonate
Marble, limestone, chalk, and calcite are examples of natural calcite. It's made by dissolving marble or limestone in $\mathrm{HCl}$, eliminating any iron or aluminium, precipitating with $\mathrm{NH}_{3}$, and adding $\left(\mathrm{NH}_{4}\right)_{2} \mathrm{CO}_{3}$to the solution.
$\mathrm{CaCl}_{2}+\left(\mathrm{NH}_{4}\right)_{2} \mathrm{CO}_{3} \longrightarrow \mathrm{CaCO}_{3}+2 \mathrm{NH}_{4} \mathrm{Cl}$
Properties
It is dissociates above 1000 degree Celsius as follows:
$\mathrm{CaCO}_{3} \longrightarrow \mathrm{CaO}+\mathrm{CO}_{2}$
It dissolves in carbon dioxide-containing water to produce calcium bicarbonate, but boiling separates it from the solution.
\[\mathrm{CaCO}_{3} \longrightarrow \mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}\;\underset{boiling} {Ca}(HCO_3)_2\]
Magnesium Carbonate
It is found in nature as magnesite, which is isomorphic to calcite. It is precipitated as a white by adding sodium bicarbonate to a solution of a magnesium salt, although only basic carbonate, known as magnesia alba, with the approximate composition $\mathrm{MgCO}_{3} . \mathrm{Mg}(\mathrm{OH})_{2} .3 \mathrm{H}_{2} \mathrm{O}$, is precipitated.
Properties: The properties of magnesium carbonate is same as calcium carbonate.
Bicarbonates
Sodium Bicarbonates
Preparation: By absorption of $\mathrm{CO}_{2}$ in $\mathrm{Na}_{2} \mathrm{CO}_{3}$ solution.
$\mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2} \rightleftharpoons 2 \mathrm{NaHCO}_{3}$
Uses: It is used in pharmaceutical industries and also as baking powder.
Potassium bicarbonates
Preparation: Same as $\mathrm{NaHCO}_{3}$
Properties: Same as $\mathrm{NaHCO}_{3}$
But it is more alkaline and more soluble in water compared to $\mathrm{NaHCO}_{3}$.
Magnesium Bicarbonate
$\mathrm{MgCO}_{3}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O} \stackrel{\text { boiling }}{\rightleftharpoons} \mathrm{Mg}\left(\mathrm{HCO}_{3}\right)_{2}$
Calcium Bicarbonate
$\mathrm{CaCO}_{3}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}$
Chlorides
Sodium Chloride
It is prepared by the method of brine which contains 25% sodium chloride.
Properties
It is nonhygroscopic, whereas ordinary salt contains magnesium chloride, which makes it hygroscopic.
It's used to make freezing mixture in the lab (freezing mixture is ice-common salt mixture with a temperature of-25 degrees Celsius).
To melt ice and snow from the road.
Potassium Chloride
It also occurs in nature as sylvite $(\mathrm{KCl})$ or carnallite $\mathrm{KCl} . \mathrm{MgCl}_{2} .6 \mathrm{H}_{2} \mathrm{O}$.
Uses: Potassium chloride is used as a fertiliser.
Magnesium Chloride
Preparation: It is prepared by dissolving $\mathrm{MgCO}_{3}$ in dilute hydrochloric acid.
$\mathrm{MgCO}_{3}+2 \mathrm{HCl} \longrightarrow \mathrm{MgCl}_{2}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}$
Properties
It crystallises as hexahydrate. $\mathrm{MgCl}_{2} \cdot 6 \mathrm{H}_{2} \mathrm{O}$.
It is deliquescent solid.
This hydrate undergoes hydrolysis as follows :
$\mathrm{MgCl}_{2}+\mathrm{H}_{2} \mathrm{O} \longrightarrow \mathrm{Mg}(\mathrm{OH}) \mathrm{Cl}+\mathrm{HCl}$
$\mathrm{Mg}(\mathrm{OH}) \mathrm{Cl} \longrightarrow \mathrm{MgO}+\mathrm{HCl}$
Anhydrous $\mathrm{MgCl}_{2}$ can be prepared by heating a double salt like. $\mathrm{MgClO}_{2} . \mathrm{NH}_{4} \mathrm{Cl} .6 \mathrm{H}_{2} \mathrm{O}$ as follows:
$MgC{l_2}.N{H_4}C{l_2}.6{H_2}O\xrightarrow[\Delta ]{{ - {H_2}O}}MgC{l_2}N{H_4}Cl\xrightarrow[\Delta ]{{strong}}MgC{l_2} + N{H_3} + HCl$
Sorel Cement: It's a paste-like mixture of magnesium oxide and magnesium chloride that hardens when left to stand. This is utilised in dental fillings, flooring, and other applications.
Calcium Chloride
In the solvay process, it is a by-product.
The carbonate can alternatively be made by dissolving it with hydrochloric acid.
$\mathrm{CaCO}_{3}+2\mathrm{HCl}\longrightarrow\mathrm{CaCl}_{2}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}$
Properties
It's made out of deliquescent crystals.
It gets hydrolysed like $\mathrm{MgCl}_{2}$ hence anhydrous $\mathrm{CaCl}_{2}$ cannot be prepared.
$\mathrm{CaCl}_{2}+\mathrm{H}_{2}\mathrm{O}\rightleftharpoons\mathrm{CaO}+2 \mathrm{HCl}$
Anhydrous $\mathrm{CaCl}_{2}$ is used in drying gases and organic compounds but not $\mathrm{NH}$ or alcohol due to the formation of $\mathrm{CaCl}_{2} .8 \mathrm{NH}_{3}$ and $\mathrm{CaCl}_{2} \cdot 4 \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}$
Sulphates
Sodium Sulphate
Preparation
It is created by heating common salt with sulphuric acid in the first step of the leblanc process.
$2 \mathrm{NaCl}+\mathrm{H}_{2} \mathrm{SO}_{4} \longrightarrow \mathrm{Na}_{2} \mathrm{SO}_{4}+2 \mathrm{HCl}$
Thus the salt cake formed is crystallised out from its aqueous solution as $\mathrm{Na}_{2} \mathrm{SO}_{4} .10 \mathrm{H}_{2} \mathrm{O}$. This is called as Glauber's salt.
Properties
Sodium sulphate is reduced to $\mathrm{Na}_{2} \mathrm{S}$ when it is fused with carbon.
$\mathrm{Na}_{2} \mathrm{SO}_{4}+4 \mathrm{C} \longrightarrow \mathrm{Na}_{2} \mathrm{S}+4 \mathrm{CO}$
Uses: It is used in pharmaceutical industries.
Potassium Sulphate
It's found in stassfurt potash deposits as schonite and kainite, and it's made by dissolving it in water and crystallising it. It separates as crystals from the solution, whereas $\mathrm{Na}_{2} \mathrm{SO}_{4}$ is a decahydrate.
Magnesium Sulphate
Preparation
It's made by dissolving kieserite $\mathrm{MgSO}_{4} . \mathrm{H}_{2} \mathrm{O}$ in boiling water and then crystallising the resulting hepta hydrate solution. Epsom salt is what it's called.
It is also obtained by dissolving magnesite in hot dilute $\mathrm{H}_{2} \mathrm{SO}_{4}$
$\mathrm{MgCO}_{3}+\mathrm{H}_{2}\mathrm{SO}_{4}\longrightarrow\mathrm{MgSO}_{4}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}$
It is isomorphous with $\mathrm{FeSO}_{4} \cdot 7 \mathrm{H}_{2} \mathrm{O}, \mathrm{ZnSO}_{4} \cdot 7 \mathrm{H}_{2} \mathrm{O}$
Calcium Sulphate
It occurs as anhydrite $\mathrm{CaSO}_{4}$ and as the dihydrate $\mathrm{CaSO}_{4}.2\mathrm{H}_{2}\mathrm{O}$, gypsum, alabaster or satinspar.
Properties
Calcium sulphate solubility increases till a certain point and then declines as temperature rises.
Because of its porous nature, plaster of Paris is utilised in the construction of wood.
Example 17:
$\mathrm{Mg}_{3} \mathrm{N}_{2}$ when reacted with water, givesoff $\mathrm{NH_3}$. but HCl is not obtained from $\mathrm{MgCl}$ on reaction with water at room temperature. Why?
The crystalline salts of alkaline earth metals contain more water of crystallization than corresponding alkali metal salts. Why?
Solution:
Because $\mathrm{Mg}_{3} \mathrm{N}_{2}$ is a salt of a strong base and a weak acid $\left(\mathrm{NH}_{3}\right)$ , it can be hydrolyzed.
$\mathrm{Mg}_{3} \mathrm{~N}_{2}+6 \mathrm{H}_{2} \mathrm{O}\longrightarrow 3 \mathrm{Mg}(\mathrm{OH})_{2}+2 \mathrm{NH}_{3}$
In comparison to alkali metal ions, alkaline earth metal ions have a stronger tendency to hydrate due to their small size and high nuclear charge. As a result, alkaline earth metal salts contain more crystallisation water than alkali metal salts.
Example 18: What happens when:
Beryllium carbide reacts with water.
Magnesium nitrate is heated.
Quick lime is heated in electric furnace with powdered coke.
Sodium chloride solution is added to zinc chloride solution.
Solution:
A gas named methane is evolved
$\mathrm{Be}_{2} \mathrm{C}+4 \mathrm{H}_{2} \mathrm{O} \longrightarrow 2 \mathrm{Be}(\mathrm{OH})_{2}+\mathrm{CH}_{4}$
A gas which is brown in colour, $\mathrm{NO}_{2}$, is evolved
$2 \mathrm{Mg} \mathrm{NO}_{3} \longrightarrow 2 \mathrm{MgO}+4 \mathrm{NO}_{2}+\mathrm{O}_{2}$
Calcium carbide is formed
$CaO + 3C\xrightarrow[{Arc}]{{Electric}}Ca{C_2} + CO$
A white precipitate of zinc hydroxide is evolved which forms sodium zincate on dissolving with excess of sodium hydroxide.
$\mathrm{ZnCl}_{2}+2\mathrm{NaOH}\longrightarrow\mathrm{Zn}(\mathrm{OH})_{2}+2 \mathrm{NaCl}$
$\mathrm{Zn}(\mathrm{OH})_{2}+2\mathrm{NaOH}\longrightarrow\mathrm{Na}_{2} \mathrm{ZnO}_{2}+2 \mathrm{H}_{2} \mathrm{O}$
Example 19: Draw a structure of:
$BeC{l_2}$ in vapour state
$BeC{l_2}$ in solid state
Solution:
It features a chlorobridged dimer structural bond in the vapour state. At 1000 degrees Celsius, it dissociates into a linear monomer.
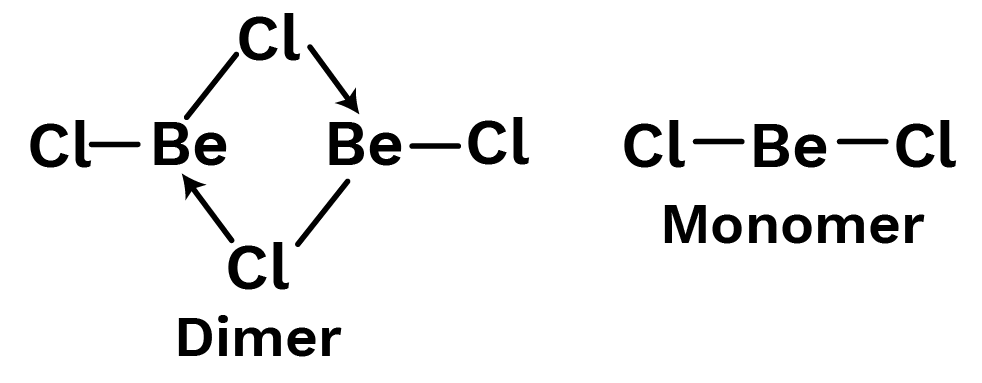
In the solid state, it has a polymeric structure with chlorobridges, in which a halogen atom connected to one beryllium atom forms a coordinate bond with a lone pair of electrons and a covalent binding with another atom.
Class 11 Chemistry Revision Notes for Chapter 10 - The s-Block Elements
About s-Block Elements Revision Notes
The s-block elements fall among the most popular and speak about the elements in chemistry. Thus in class 11, chapter 10 basically throws light on s-block elements, including their properties, characteristics, types, and the importance of different compounds. Aiming to help the students learn about these topics and study productively, a free CBSE revision notes for class 11, chapter 10 Chemistry - The s-block elements are provided at Vedantu. Students can access the revision notes here at this page and get easily accustomed to all the important topics given in the chapter. Also, students can use the revision notes at their convenience irrespective of the time or the place.
Periodic Trends in the Properties of s-block Elements
Alkali Metal - These are the metals of silvery-white, soft, low melting, and highly reactive. The ionic size and the atomic size as well increase down the group. On the other side, the ionization enthalpies decrease down the group. Mostly, these compounds are ionic. Their oxides and hydroxides dissolve in water to form strong alkalies. A few compounds of sodium are sodium hydrogen carbonate, sodium chloride, and more related. The Castner-Kellner process does the manufacturing of Sodium hydroxide, and the Solvay process achieves the manufacturing of sodium carbonate.
Alkaline Earth Metals - These are the metals having increased cationic charges. Their atomic size and the ionic size is reduced. Oxides and hydroxides of alkaline earth metals are less basic. A few of the compounds of calcium are calcium hydroxide, calcium carbonate, and more related.
Diagonal Relationship - The first element of group 1 and the second element of group 2 exhibits similar properties: the Lithium from group 1 and Beryllium from group 2. Thus, such similarities are known as diagonal relationships.
What are The s-block Elements?
The s-block of the periodic table contains Group 1 and Group 2 elements. Moreover, their hydroxides and oxides are alkaline in nature. Furthermore, they are characterized by the s- electron or electrons in their atom's valence shell. Besides, they are highly reactive metals that form mono and dipositive ions. Additionally, with the increasing atomic number, there is a regular trend in the alkali metal's physical and chemical properties.
Most notably, the first element of both Group 1 and Group 2 exhibits the same properties. In the periodic table, these similarities are referred to as the diagonal relationship. Moreover, the chemistry of alkaline earth metals is much similar to the alkali metals. Furthermore, there are some differences because of the ionic and atomic sizes.
Sub-Topics of The s- Block Elements
Let us look at the subtopics that fall under the topic, The s-block elements:
Anomalous Behaviour of Lithium - This topic explains about lithium, its nature, and similarities between the lithium and magnesium, including the difference between lithium with other alkali metals.
Beryllium, Magnesium, and Calcium - This topic gives an overview of magnesium, beryllium, and calcium.
Characteristics of the Compounds of Alkali Earth Metals - This topic speaks about the alkali earth metals, including their physical and chemical properties.
Characteristics of the Compounds of Alkali Metals - The topic describes the characteristics of compounds of alkali metals and also discusses a few other alkali metals.
Group 1 Elements, Alkali Metals - This topic highlights the group1 elements like sodium, lithium, potassium, and more.
Group 2 Elements, Alkali Earth Metals - This topic teaches us about the group 2 elements such as calcium, magnesium, and more related.
Some Important Compounds of Potassium and Sodium - This topic defines the important compound uses and sodium and potassium properties.
Why Choose Vedantu?
Vedantu is one of the biggest online learning platforms where students can get many benefits, where some of them are mentioned below:
Students can be updated on the latest information regarding their respective state boards
They can get various info on question papers, revision notes, last 5 years question papers, information on specific topics, and a lot more
By going through all these academic papers, students can gain a better understanding of every topic or chapter
They can also clear the doubts on a specific topic or chapter
Even the students can download and save these papers and keep with them, which can be used further at the time of their examinations
FAQs on CBSE Notes Class 11 Chemistry Chapter 10 - The S Block Elements - 2025-26
1. What are the key topics I should focus on when revising The s-Block Elements for Class 11?
For a quick and effective revision of The s-Block Elements, you should focus on the following core areas:
- Group 1 (Alkali Metals): Their electronic configuration, trends in atomic and ionic radii, ionisation enthalpy, and hydration enthalpy. Pay special attention to the anomalous properties of lithium and its diagonal relationship with magnesium.
- Group 2 (Alkaline Earth Metals): Similar trends as Group 1, but compare their properties like higher melting points and ionisation enthalpies. Focus on the anomalous behaviour of beryllium and its diagonal relationship with aluminium.
- Important Compounds: Understand the preparation, properties, and uses of key compounds like Sodium Carbonate (Washing Soda) via the Solvay process, Sodium Hydroxide (Caustic Soda), and Calcium Sulphate (Plaster of Paris).
- Chemical Reactivity: Revise reactions with air (formation of oxides, peroxides, superoxides), water, hydrogen, and halogens for both groups.
2. Why are Group 1 and Group 2 elements called Alkali Metals and Alkaline Earth Metals respectively?
Group 1 elements are called Alkali Metals because they react with water to form strong hydroxides, which are highly alkaline in nature. The term 'alkali' is derived from the Arabic word 'al-qaly', meaning plant ashes, from which compounds of sodium and potassium were first isolated. Group 2 elements are called Alkaline Earth Metals because their oxides and hydroxides are also alkaline in nature and these metal oxides were found in the earth's crust and were stable to heat.
3. How do key atomic properties like ionisation enthalpy and atomic radii trend down the groups in s-block elements?
When revising trends for s-block elements, remember these two key points for both Group 1 and Group 2:
- Atomic and Ionic Radii: The size of the atoms and their corresponding ions increases as you move down the group. This is because a new electron shell is added for each successive element.
- Ionisation Enthalpy: The energy required to remove the outermost electron decreases down the group. As the atomic size increases, the outermost electron is further from the nucleus and is shielded by inner electrons, making it easier to remove.
4. What is the anomalous behaviour of lithium, and why does it show a diagonal relationship with magnesium?
Lithium, the first member of Group 1, shows anomalous behaviour due to its exceptionally small atomic size and high polarising power. This leads to increased covalent character in its compounds. Key differences from other alkali metals include its hardness, higher melting point, and formation of only monoxide (Li₂O) and nitride (Li₃N) on reacting with air.
Lithium shows a diagonal relationship with magnesium (Group 2) because they have a similar charge-to-radius ratio. Their similarities include:
- Both are harder than other metals in their respective groups.
- Both react directly with nitrogen to form nitrides.
- Their carbonates decompose on heating to form oxides and CO₂.
- Their chlorides are deliquescent and soluble in ethanol.
5. Why does beryllium show properties different from other alkaline earth metals?
Beryllium shows anomalous behaviour compared to other Group 2 elements primarily due to its extremely small atomic size and high ionisation enthalpy. This gives it a high polarising power, leading to its compounds being largely covalent, unlike the ionic compounds of other alkaline earth metals. For instance, beryllium oxide (BeO) is amphoteric, while the oxides of other group members are basic. It also shows a diagonal relationship with aluminium (Al) in Group 13.
6. What are the essential steps to remember for the Solvay process for preparing sodium carbonate?
To revise the Solvay process, focus on these main steps: First, a brine solution (concentrated NaCl) is saturated with ammonia. This ammoniated brine is then carbonated by passing carbon dioxide through it, which leads to the precipitation of sodium bicarbonate (NaHCO₃) because it is sparingly soluble in the cold solution. Finally, the separated sodium bicarbonate is heated (calcination) to produce sodium carbonate (Na₂CO₃).
7. Why can't potassium carbonate be prepared by the Solvay process?
Potassium carbonate (K₂CO₃) cannot be prepared by the Solvay process due to a key difference in solubility. In the process, the crucial step is the precipitation of bicarbonate. While sodium bicarbonate (NaHCO₃) is sparingly soluble and precipitates out from the ammoniated brine solution, potassium bicarbonate (KHCO₃) is too soluble in water. This high solubility prevents it from precipitating, making the separation and subsequent conversion to potassium carbonate unfeasible through this method.
8. How do the oxides formed by alkali metals differ, and what determines the type of oxide formed?
The type of oxide formed by alkali metals upon heating in excess air depends on the size and stability of the cation.
- Lithium (Li), being small, forms normal oxide, Li₂O.
- Sodium (Na) forms the peroxide, Na₂O₂.
- Potassium (K), Rubidium (Rb), and Cesium (Cs), being larger, can stabilise the larger superoxide ion and form superoxides (KO₂, RbO₂, CsO₂).
9. What is the chemical principle behind Plaster of Paris setting into a hard mass?
The setting of Plaster of Paris is a process of hydration. Plaster of Paris is calcium sulphate hemihydrate (CaSO₄·½H₂O). When mixed with water, it re-forms gypsum (CaSO₄·2H₂O), a hard, solid mass. This reaction is exothermic and involves the interlocking of gypsum crystals, which gives the set plaster its rigidity and strength.
10. The second ionisation enthalpy of alkaline earth metals is very high, yet they form M²⁺ ions. Why is this energetically favourable?
Although the second ionisation enthalpy (removing a second electron) for alkaline earth metals is significantly higher than the first, the formation of a dipositive ion (M²⁺) is ultimately favourable in compounds. This is because the high energy required is more than compensated by the very high lattice enthalpy (in solid state) or hydration enthalpy (in aqueous solution) released. The small size and high charge of the M²⁺ ion lead to strong attractions, making the overall process energetically favourable for forming compounds like CaCl₂ rather than CaCl.
11. How can I quickly recall which s-block elements give a characteristic colour in a flame test and why some do not?
Most s-block elements impart a characteristic colour to a flame. When heated, the valence electrons get excited to higher energy levels and emit light of a specific colour upon returning to the ground state. You can remember the colours as:
- Lithium: Crimson Red
- Sodium: Golden Yellow
- Potassium: Lilac (Pale Violet)
- Rubidium: Reddish Violet
- Cesium: Blue
- Calcium: Brick Red
- Strontium: Crimson Red
- Barium: Apple Green
12. Why are alkali metal solutions in liquid ammonia blue and highly conducting?
When alkali metals dissolve in liquid ammonia, they form a deep blue solution that is highly conductive. This happens because the alkali metal atom loses its valence electron. Both the cation (M⁺) and the electron become surrounded by ammonia molecules, a process called solvation. The blue colour of the solution is due to the presence of these ammoniated electrons, which absorb light in the visible region. The high electrical conductivity is also due to these mobile ammoniated electrons as well as the ammoniated cations.



















