
Write the reactions involved in Gabriel Phthalimide synthesis.
Answer
573.6k+ views
Hint: Let us first understand about Gabriel Phthalimide reaction. It is a chemical reaction in which the primary alkalyhallides are transformed into primary amines. Generally this reaction uses potassium phthalimide. This chemical reaction is named after the German chemist Siegmund Gabriel.
Complete Step by step answer: Gabriel Phthalimide synthesis is the chemical reaction that is involved in the preparation of amines. When potassium hydroxide reacts with phthalimide, a neulceophile in the form of an imide ion is formed. The imide ion along with alkyl halide undergoes a nucleophilic substitution reaction that forms an intermediate N-alkyl phthalimide. This intermediate phthalimide further undergoes hydrolysis or hydrazinolysis to yield a primary alkyl amine. Basically, the Gabriel reaction involves the alkylation of sulfonamides and amides that is followed by deprotection to obtain primary amines.
Steps involved in Gabriel Phthalimide synthesis:
-The potassium or sodium salts of phthalimide are N-alkylated with the primary alkyl halide to obtain the corresponding N-alkyl phthalimide.
-The phthalimide obtained by alkylation is not a nucleophile and hence does not react further. Therefore hydrolyzation of this intermediate phthalimide is necessary.
-Further, the alkyl phthalimide obtained undergoes hydrolysis to obtain primary amine.
Gabriel Phthalimide reaction:
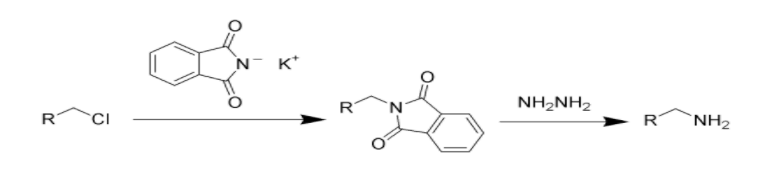
Note: The aryl amines cannot be prepared by Gabriel phthalimide synthesis because aryl halides do not undergo simple nucleophilic substitution. The huge benefit of Gabriel phthalimide synthesis is that it avoids over alkylation.
Complete Step by step answer: Gabriel Phthalimide synthesis is the chemical reaction that is involved in the preparation of amines. When potassium hydroxide reacts with phthalimide, a neulceophile in the form of an imide ion is formed. The imide ion along with alkyl halide undergoes a nucleophilic substitution reaction that forms an intermediate N-alkyl phthalimide. This intermediate phthalimide further undergoes hydrolysis or hydrazinolysis to yield a primary alkyl amine. Basically, the Gabriel reaction involves the alkylation of sulfonamides and amides that is followed by deprotection to obtain primary amines.
Steps involved in Gabriel Phthalimide synthesis:
-The potassium or sodium salts of phthalimide are N-alkylated with the primary alkyl halide to obtain the corresponding N-alkyl phthalimide.
-The phthalimide obtained by alkylation is not a nucleophile and hence does not react further. Therefore hydrolyzation of this intermediate phthalimide is necessary.
-Further, the alkyl phthalimide obtained undergoes hydrolysis to obtain primary amine.
Gabriel Phthalimide reaction:

Note: The aryl amines cannot be prepared by Gabriel phthalimide synthesis because aryl halides do not undergo simple nucleophilic substitution. The huge benefit of Gabriel phthalimide synthesis is that it avoids over alkylation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




