
Write the monomers of the following polymer:
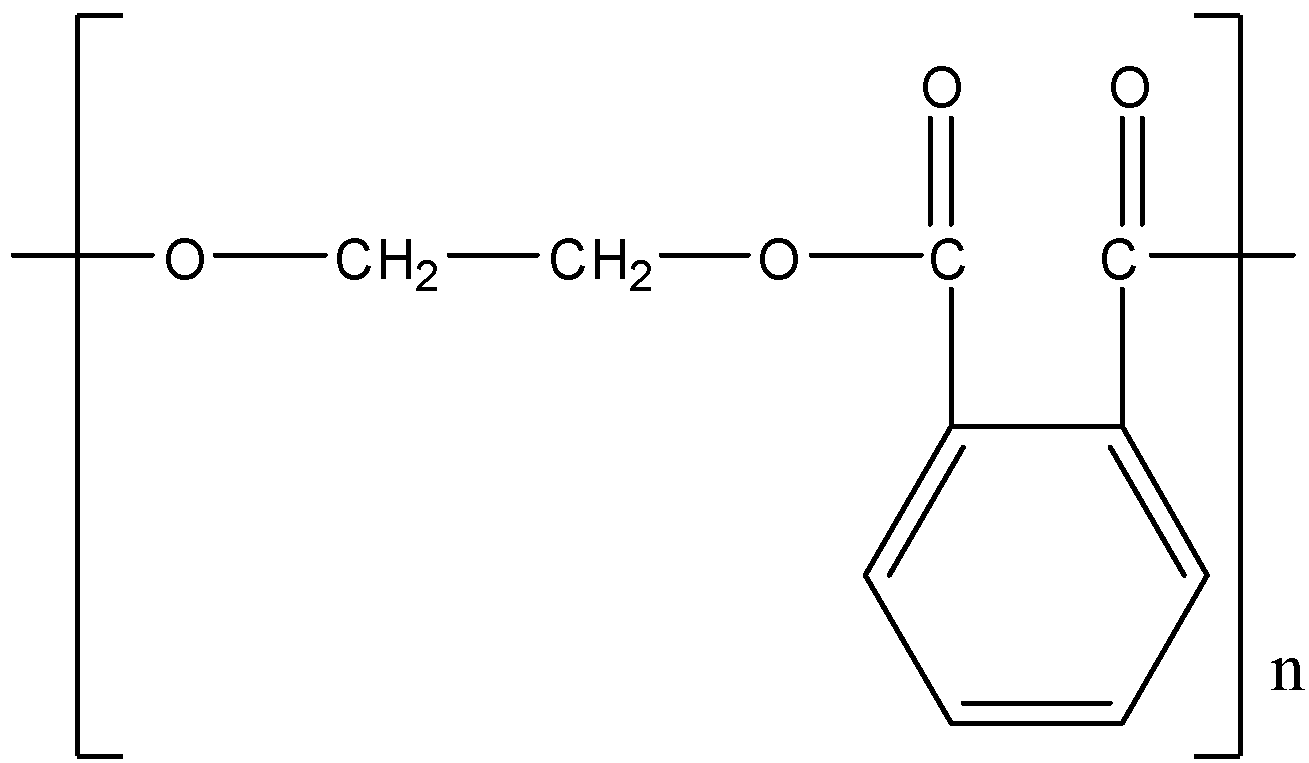

Answer
594k+ views
Hint: Polymer is macromolecule formed by joining together through covalent bonds, a large number of simple repeating structural units derived from molecules called monomers.
Complete answer:
We have been given a polymer showing one repeating structural unit. We can determine the structural formula of the monomers from which this repeating unit has been formed.
It can be inferred from the figure that this repeating unit has been formed by the condensation of two molecules. There is an ester linkage ($-COO-C{{H}_{2}}-$) between two units. We know that esters are formed by the reaction of carboxylic acids with alcohol.
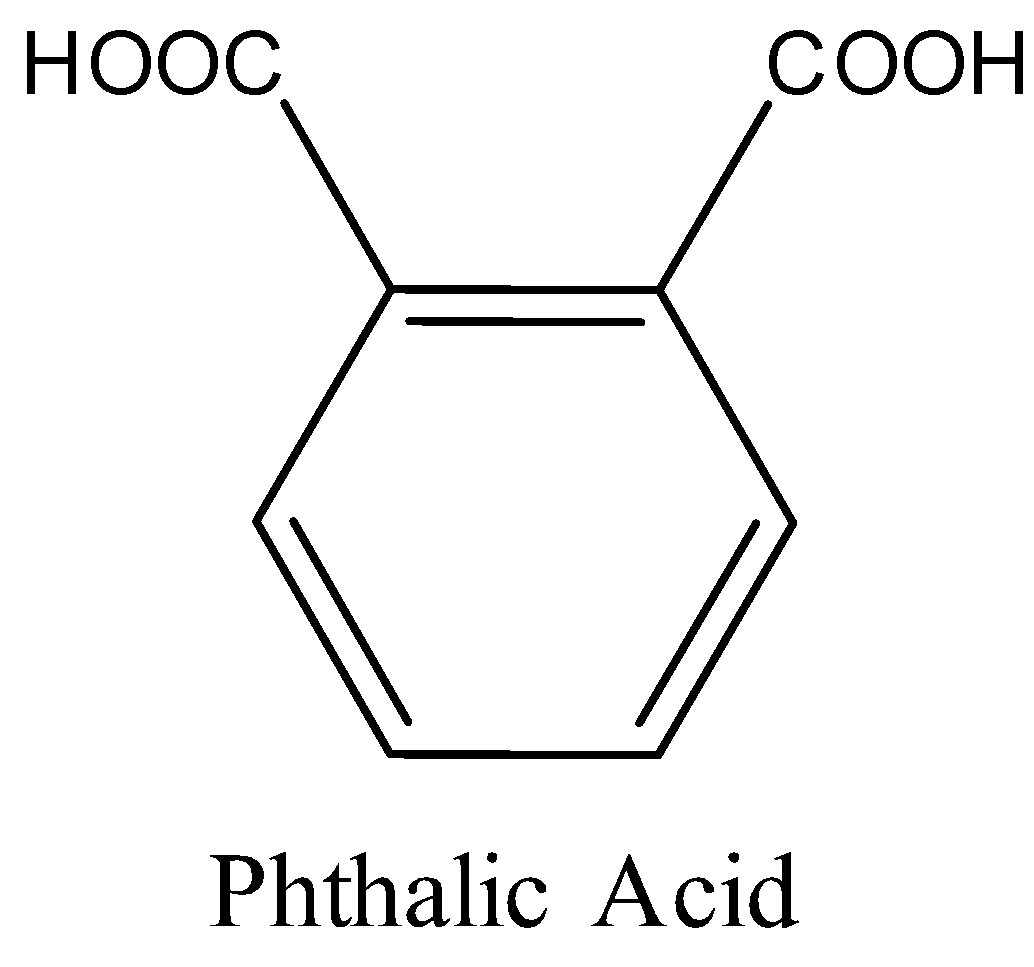
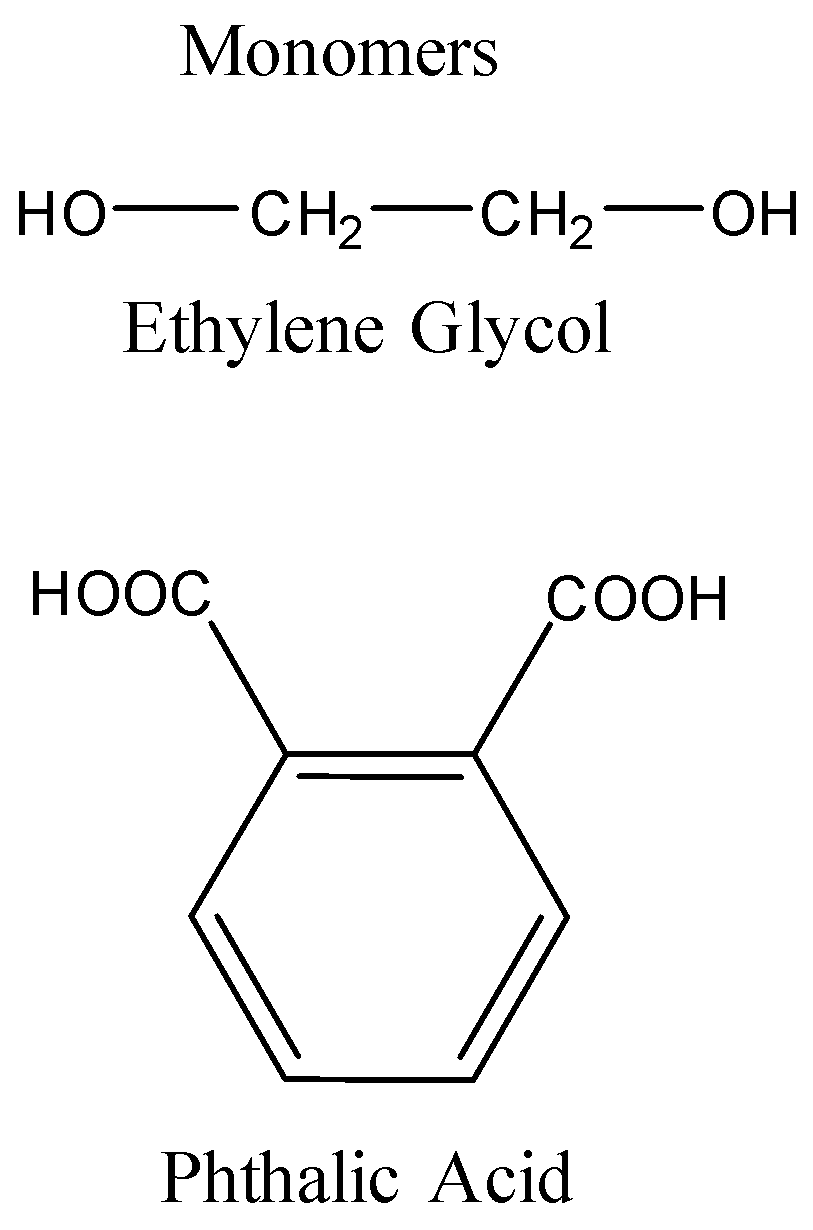
Thus, given polymer is polyester, which is formed by the polymerization of phthalic acid and dihydric alcohol, i.e., ethylene glycol with the removal of water as a byproduct.

The polymer thus formed is called poly (ethylene phthalate) or glyptal.
Hence, the monomers of the given polymer, i.e. glyptal are phthalic acid and ethylene glycol.
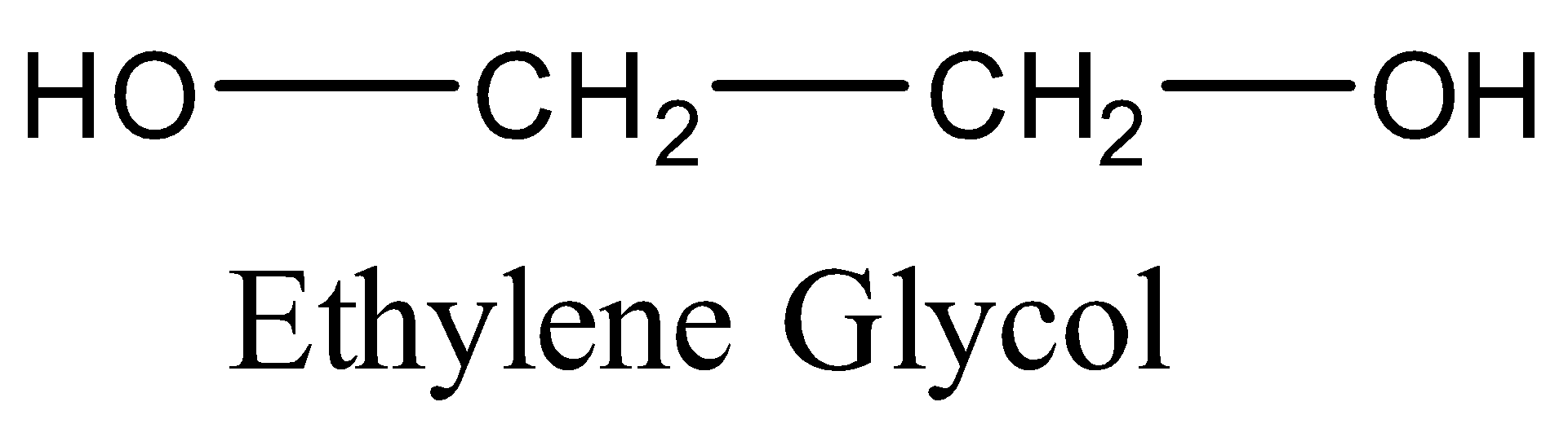
Additional Information:
Glyptal is a thermoplastic. When dissolved in suitable solvents, it forms a solution which evaporates from a tough, inflexible film. This property of glyptal is exploited to manufacture paints and lacquers.
Note: We use terms monomers and repeating structural unit interchangeably but these are actually different. Repeating structural units are joined together to form a polymer whereas monomers are the molecules from which repeating units are derived.
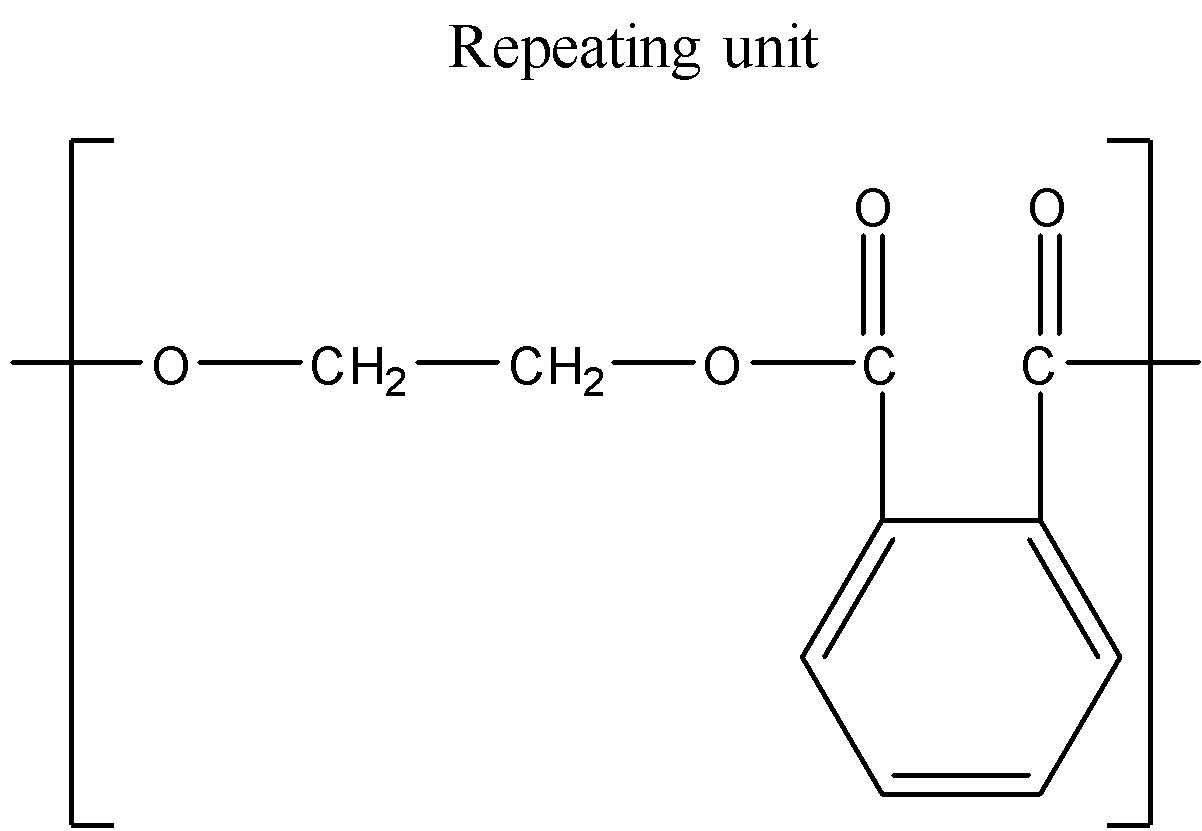
Complete answer:
We have been given a polymer showing one repeating structural unit. We can determine the structural formula of the monomers from which this repeating unit has been formed.
It can be inferred from the figure that this repeating unit has been formed by the condensation of two molecules. There is an ester linkage ($-COO-C{{H}_{2}}-$) between two units. We know that esters are formed by the reaction of carboxylic acids with alcohol.
Thus, given polymer is polyester, which is formed by the polymerization of phthalic acid and dihydric alcohol, i.e., ethylene glycol with the removal of water as a byproduct.

The polymer thus formed is called poly (ethylene phthalate) or glyptal.
Hence, the monomers of the given polymer, i.e. glyptal are phthalic acid and ethylene glycol.
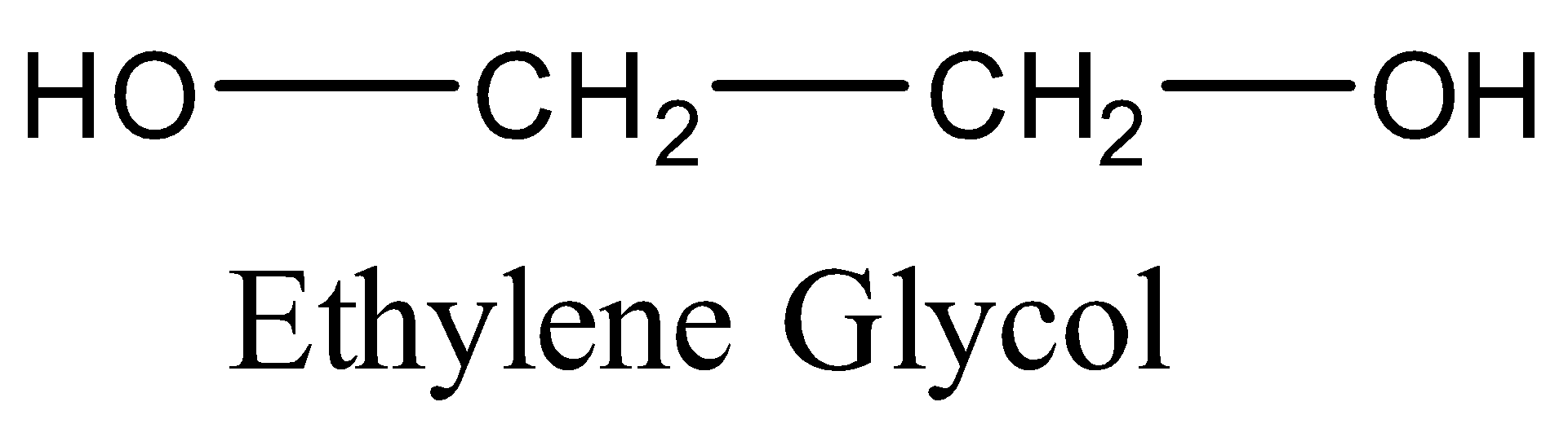

Additional Information:
Glyptal is a thermoplastic. When dissolved in suitable solvents, it forms a solution which evaporates from a tough, inflexible film. This property of glyptal is exploited to manufacture paints and lacquers.
Note: We use terms monomers and repeating structural unit interchangeably but these are actually different. Repeating structural units are joined together to form a polymer whereas monomers are the molecules from which repeating units are derived.


Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




