
Which type of alcohol Cyclohexanol is:
(A) ${{3}^{\circ }}$
(B) ${{2}^{\circ }}$
(C) ${{1}^{\circ }}$
(D) none of the given
Answer
573.9k+ views
Hint: The alcohols are classified on the basis of the attachment of carbon atoms. These are classified as primary, secondary and tertiary depending upon the attached to the primary, secondary, or tertiary carbon atom.
Complete step by step solution:
We have been provided with cyclohexanol,
We need to tell under which category of alcohols does it belongs:
So, for that:
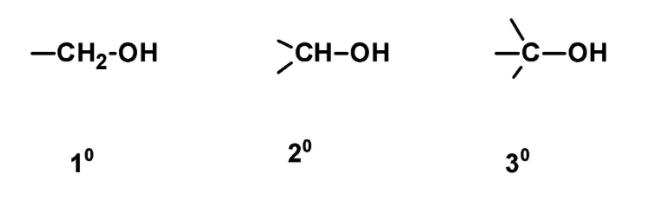
The carbon atoms are classified based on the attachment of the carbon with the adjacent group. These are classified as primary $\text{ }{{\text{1}}^{\text{o }}}$ , secondary $\text{ }{{\text{2}}^{\text{o }}}$, and tertiary $\text{ }{{\text{3}}^{\text{o }}}$. The primary is single carbon, two carbon, and the three carbon atoms respectively.
Alcohols are compounds that contain a hydroxyl $\text{ }-\text{OH }$ group. It has a single hydroxyl group as the substituent.
To determine the type of alcohol we will first start with the cyclohexane. Cyclohexane is a six-membered carbon ring. Each carbon atom is bonded to the adjacent two carbon atoms. Thus, every carbon atom in the cyclohexane is secondary $\text{ }{{\text{2}}^{\text{o }}}$.

Now, cyclohexanol:

In cyclohexanol, the hydroxyl $\text{ }-\text{OH }$group is attached to one of the carbon atoms of the cyclohexane. Thus, the carbon atom bearing a hydroxyl group is secondary carbon. Thus, cyclohexanol is a secondary alcohol. So, the correct answer is “Option B”.
Note: cyclohexanol can affect us when inhaled or by passing through your skin. Its Contact can irritate and burn the skin and eyes. Breathing Cyclohexanol can irritate the nose and throat. High exposure to cyclohexanol may cause headaches, nausea, vomiting, dizziness, and passing out.

Complete step by step solution:
We have been provided with cyclohexanol,
We need to tell under which category of alcohols does it belongs:
So, for that:
The carbon atoms are classified based on the attachment of the carbon with the adjacent group. These are classified as primary $\text{ }{{\text{1}}^{\text{o }}}$ , secondary $\text{ }{{\text{2}}^{\text{o }}}$, and tertiary $\text{ }{{\text{3}}^{\text{o }}}$. The primary is single carbon, two carbon, and the three carbon atoms respectively.
Alcohols are compounds that contain a hydroxyl $\text{ }-\text{OH }$ group. It has a single hydroxyl group as the substituent.
To determine the type of alcohol we will first start with the cyclohexane. Cyclohexane is a six-membered carbon ring. Each carbon atom is bonded to the adjacent two carbon atoms. Thus, every carbon atom in the cyclohexane is secondary $\text{ }{{\text{2}}^{\text{o }}}$.

Now, cyclohexanol:

In cyclohexanol, the hydroxyl $\text{ }-\text{OH }$group is attached to one of the carbon atoms of the cyclohexane. Thus, the carbon atom bearing a hydroxyl group is secondary carbon. Thus, cyclohexanol is a secondary alcohol. So, the correct answer is “Option B”.
Note: cyclohexanol can affect us when inhaled or by passing through your skin. Its Contact can irritate and burn the skin and eyes. Breathing Cyclohexanol can irritate the nose and throat. High exposure to cyclohexanol may cause headaches, nausea, vomiting, dizziness, and passing out.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




