
which one of the following structures represents the neoprene polymer?
(a)
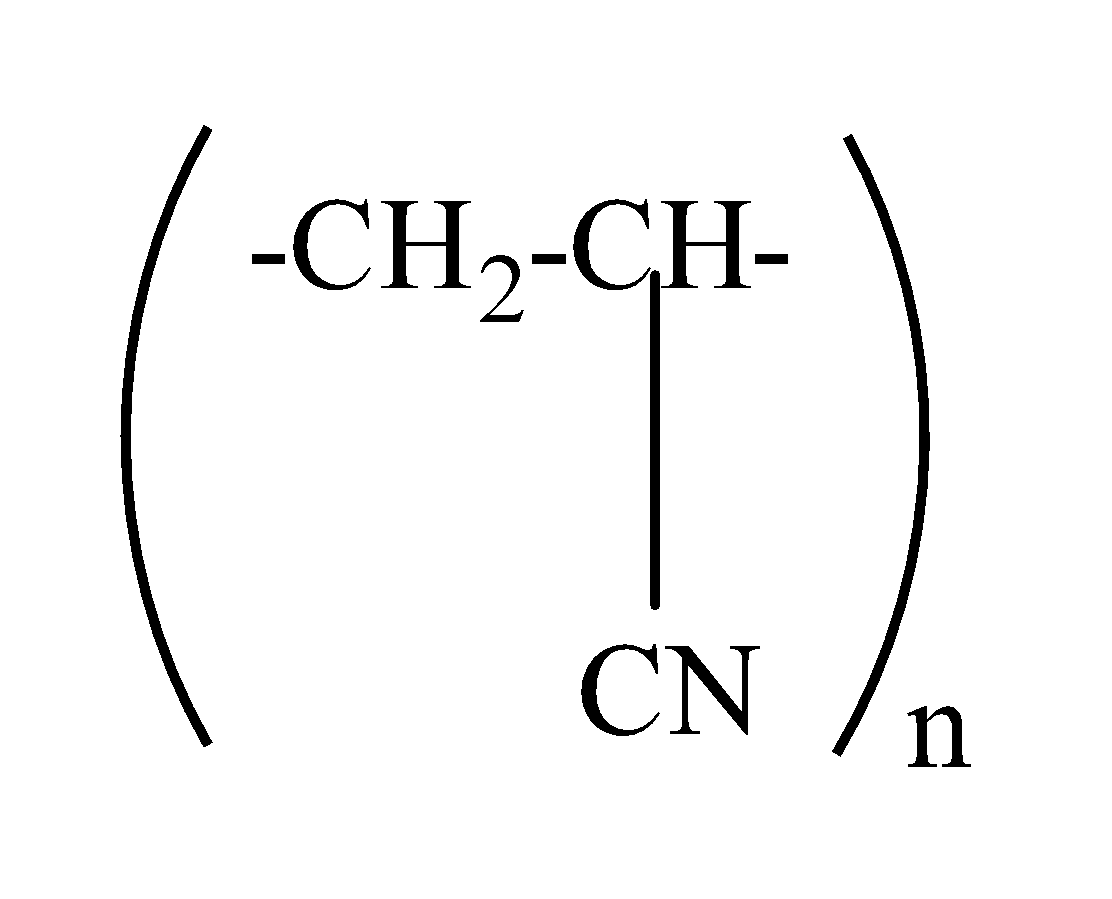
(b)
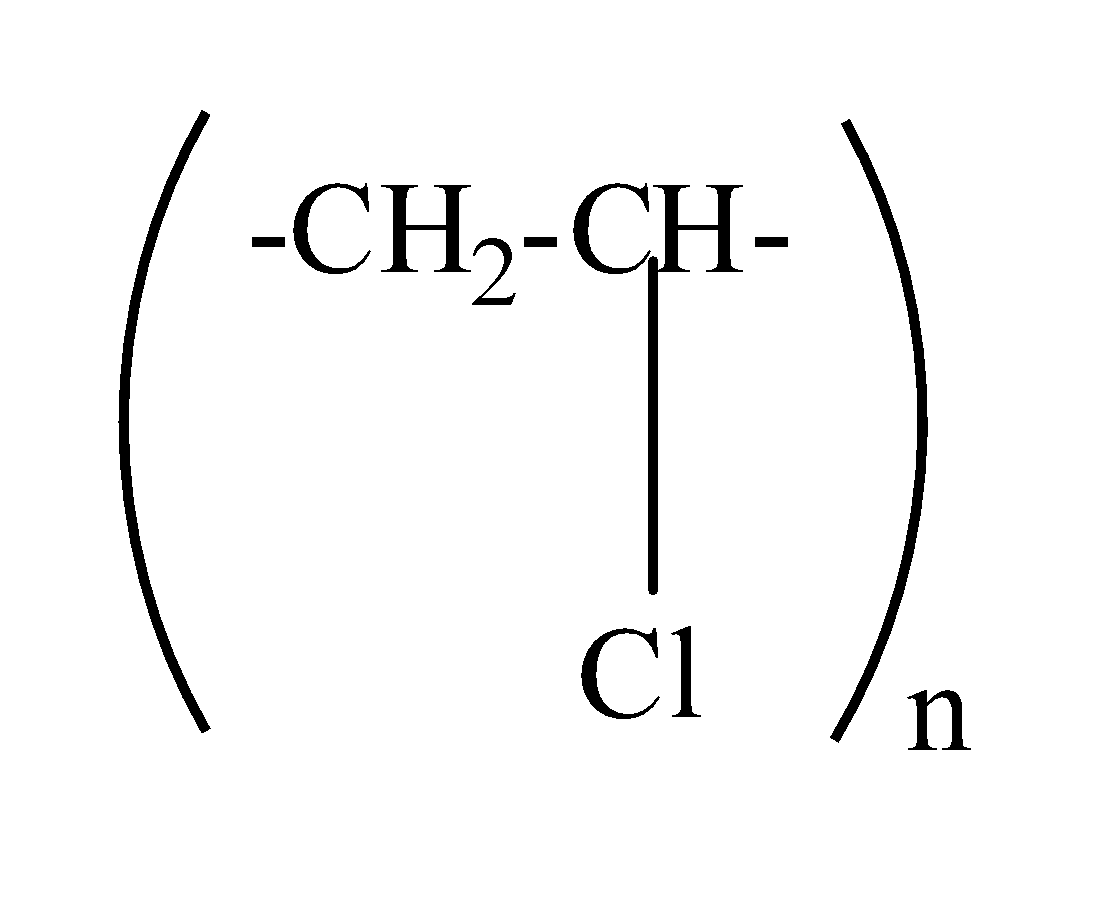
(c)
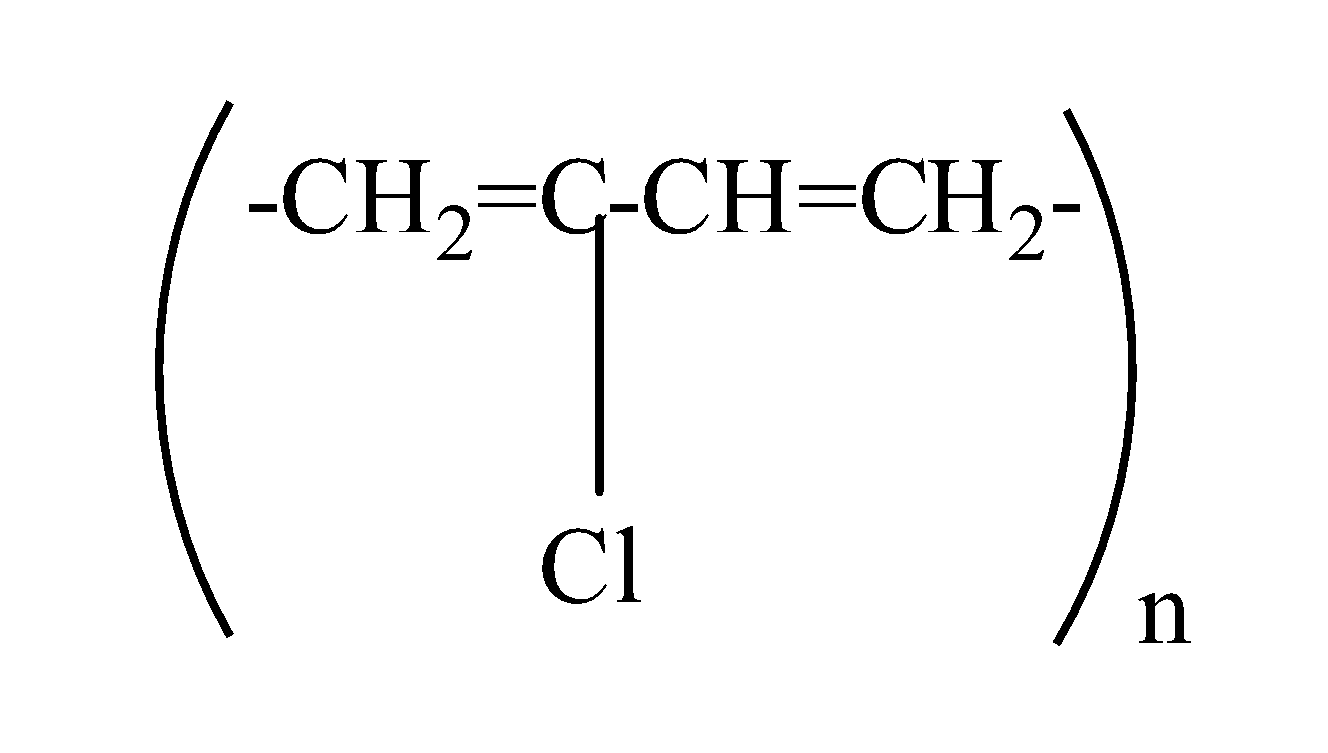
(d)
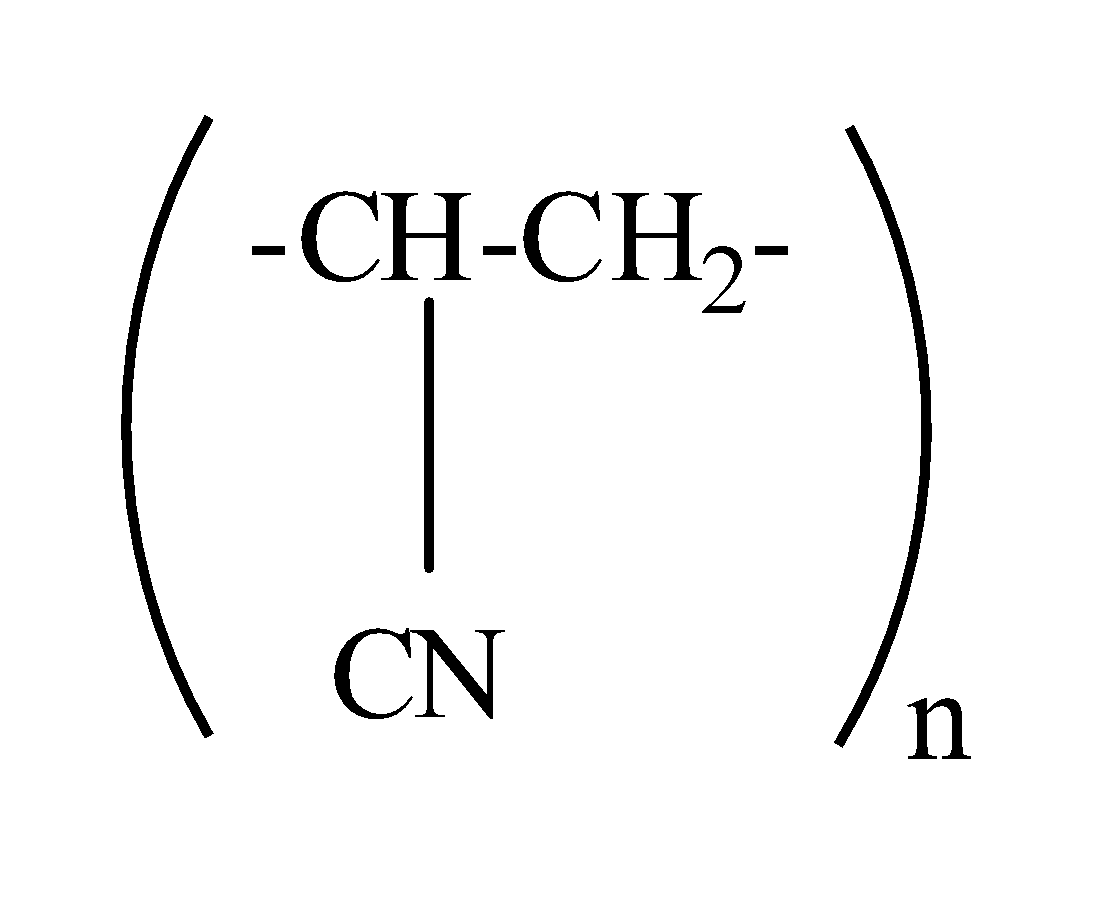




Answer
552.3k+ views
Hint: Neoprene comes under the category of synthetic rubber and is formed by the polymerization of the free radical of 2-chlorobutan-1,3-diene. Now you can easily answer the given statement accordingly.
Complete step by step answer:
First of all, we should know what polymers actually are. Polymers are the high molecular mass compounds, obtained by joining together a larger number of simple molecules through covalent bonds in a regular manner. And the simple molecules which combine to form a polymer are called the monomers and this process of formation of the polymers is known as polymerization.
- Now consider the statement as:
Neoprene is a synthetic rubber i.e. a polymer that is capable of getting stretched to about twice its length and it returns to its original shape and size as soon as the external stretching force is released. These have properties similar to rubber and some additional desirable properties.
It is prepared by the free radical polymerization of chloroprene (2-chlorobuta-1,3-diene) as:
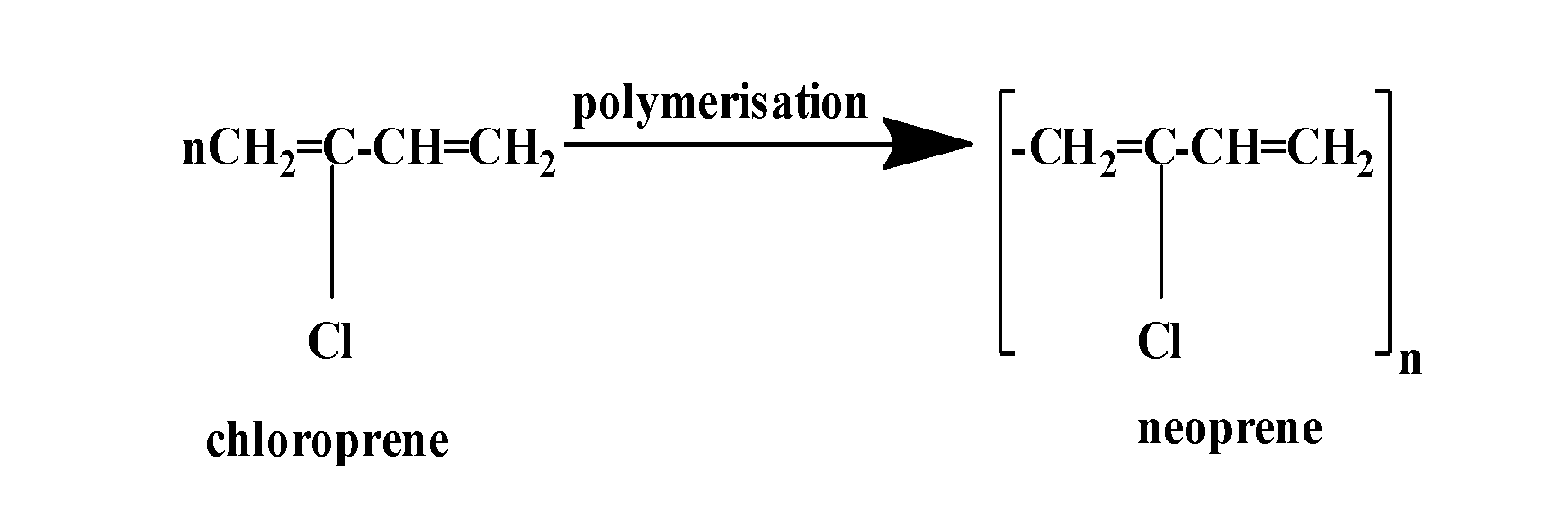
So, the correct answer is “Option D”.
Note: Neoprene is superior to natural rubber in its stability to aerial oxidation and its resistance to vegetable and mineral oils, gasoline, and other solvents. It is used as an insulator, making conveyor belts, hoses, etc.
Complete step by step answer:
First of all, we should know what polymers actually are. Polymers are the high molecular mass compounds, obtained by joining together a larger number of simple molecules through covalent bonds in a regular manner. And the simple molecules which combine to form a polymer are called the monomers and this process of formation of the polymers is known as polymerization.
- Now consider the statement as:
Neoprene is a synthetic rubber i.e. a polymer that is capable of getting stretched to about twice its length and it returns to its original shape and size as soon as the external stretching force is released. These have properties similar to rubber and some additional desirable properties.
It is prepared by the free radical polymerization of chloroprene (2-chlorobuta-1,3-diene) as:

So, the correct answer is “Option D”.
Note: Neoprene is superior to natural rubber in its stability to aerial oxidation and its resistance to vegetable and mineral oils, gasoline, and other solvents. It is used as an insulator, making conveyor belts, hoses, etc.
Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




