
Which of these tautomers is more stable?

$\text{ 1 2}$
(A) 1
(B) 2
(C) 1=2
(D) None of these

Answer
573.9k+ views
Hint: To make a correct tautomer we see the type of functional group and then we see the movement of the lone pair. When we move the lone pair and migrate the hydrogen to the correct position, we get the tautomer.
Complete step by step answer:
The above tautomerism is a functional group isomerism. In the functional group isomerism, the carbon skeleton of that compound is totally unchanged but it shows proton transfer in an intramolecular way. Since the functional group is the amine group so the tautomerism is imine-enamine isomerism.
Amines show tautomerism by getting converted to imine form. To show isomerism or tautomerism it should contain at least \[1\] alpha-hydrogen in it. This alpha-hydrogen is shifted or migrated to the nitrogen.

Enamine Imine
For stability among these two we will see the analogy between ketones and amines. In ketones the
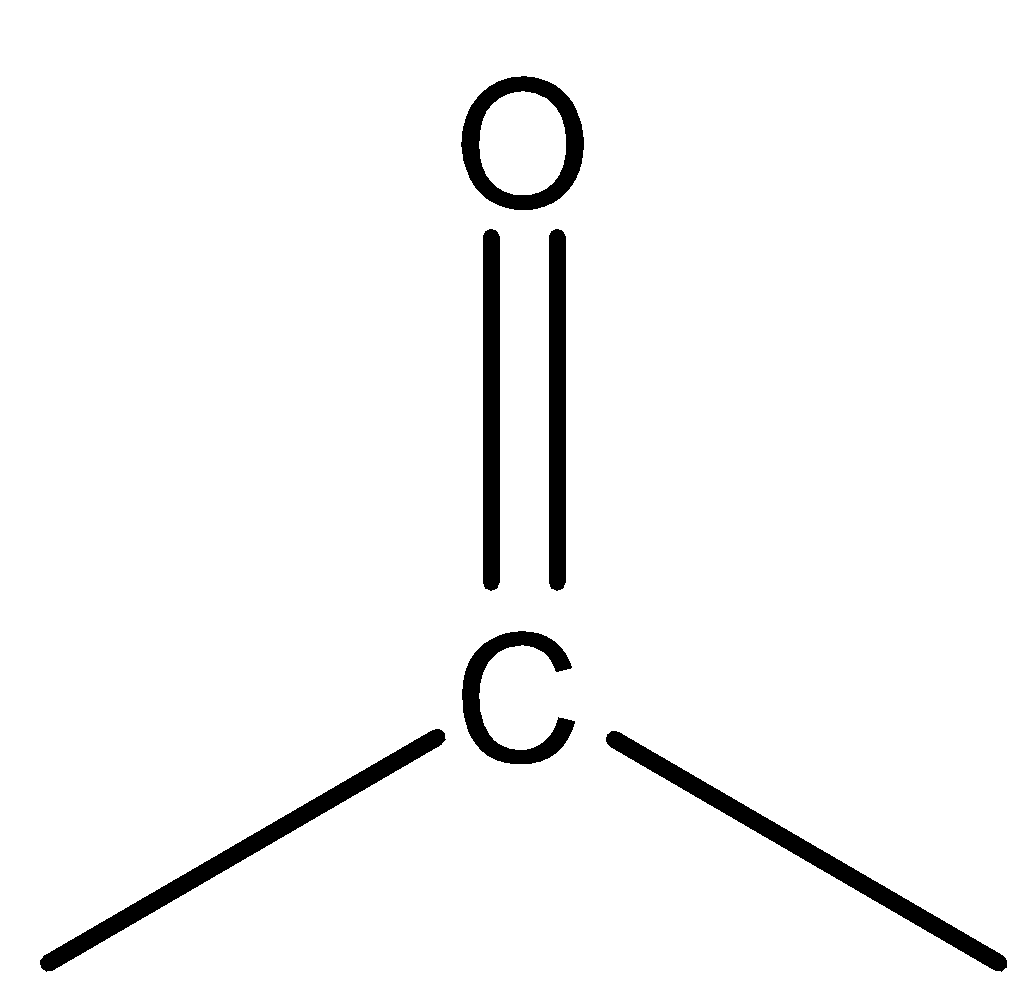 is more stable than
is more stable than
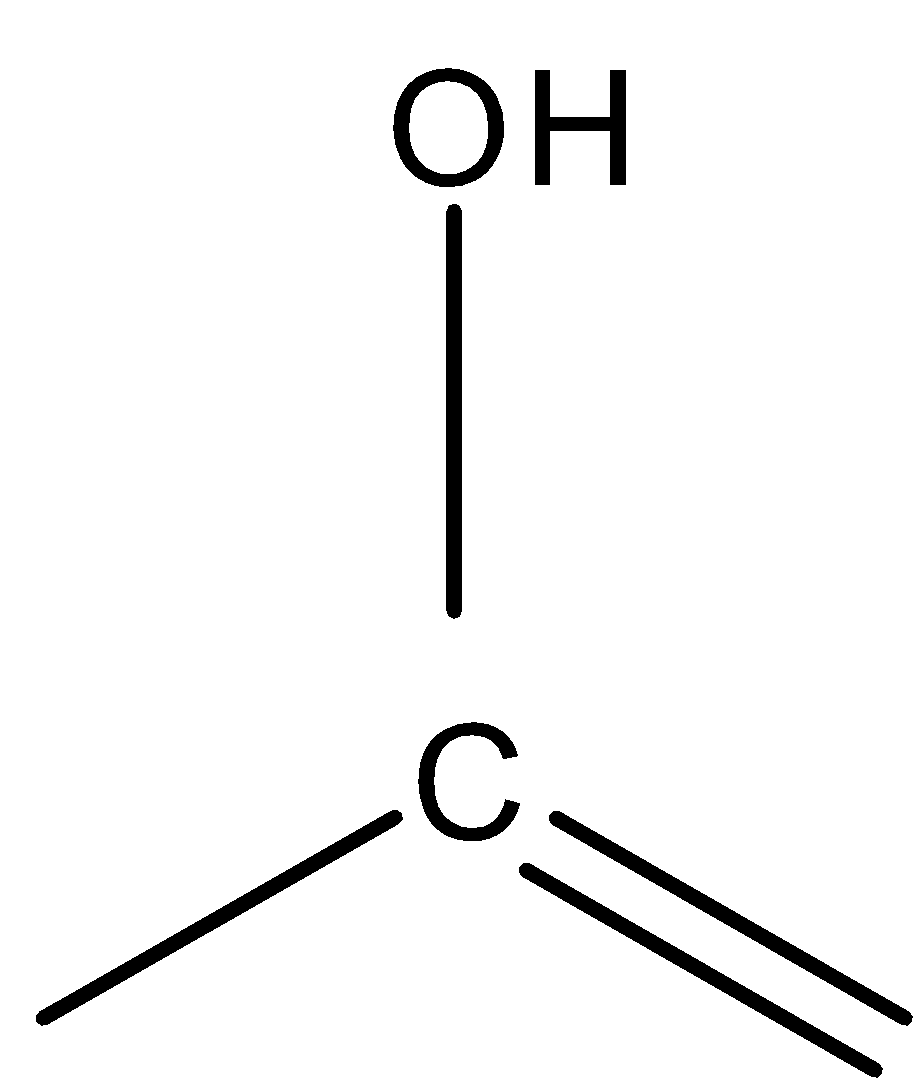 because of a stable carbon-oxygen bond. Similarly,
because of a stable carbon-oxygen bond. Similarly,
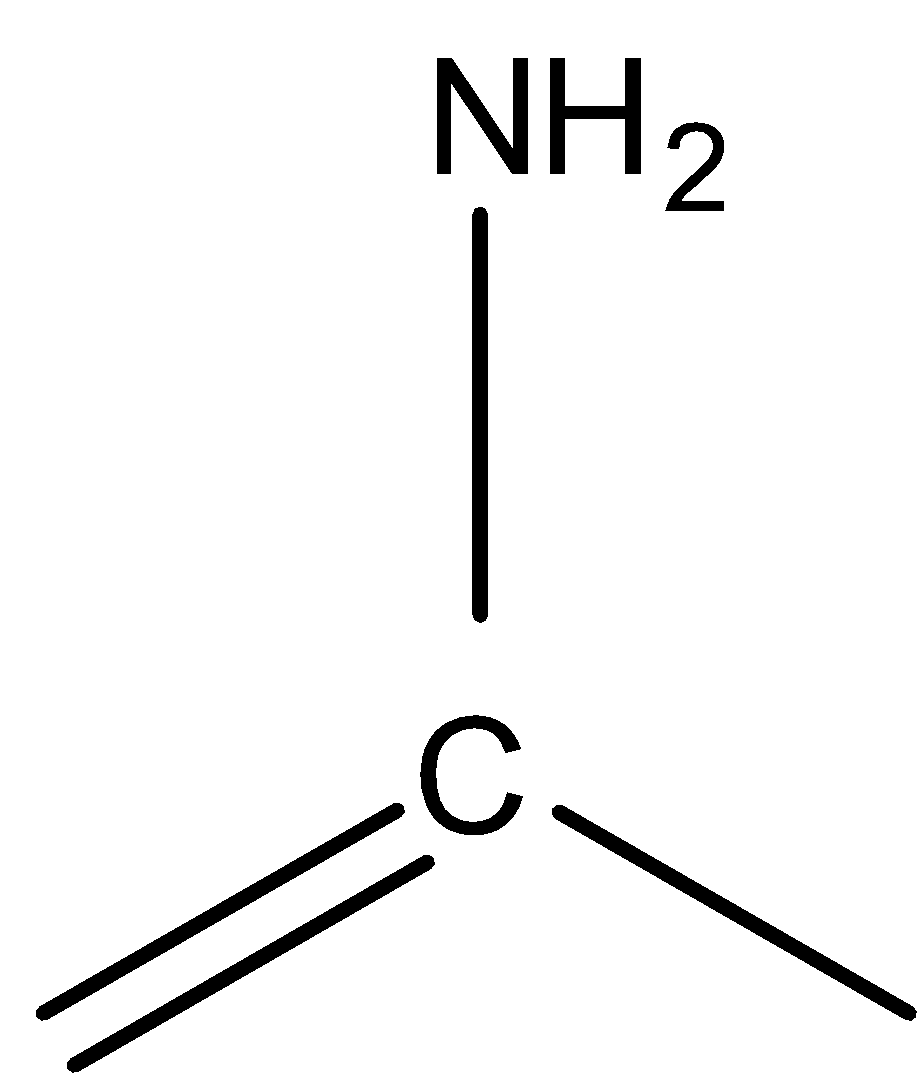 is less stable than
is less stable than
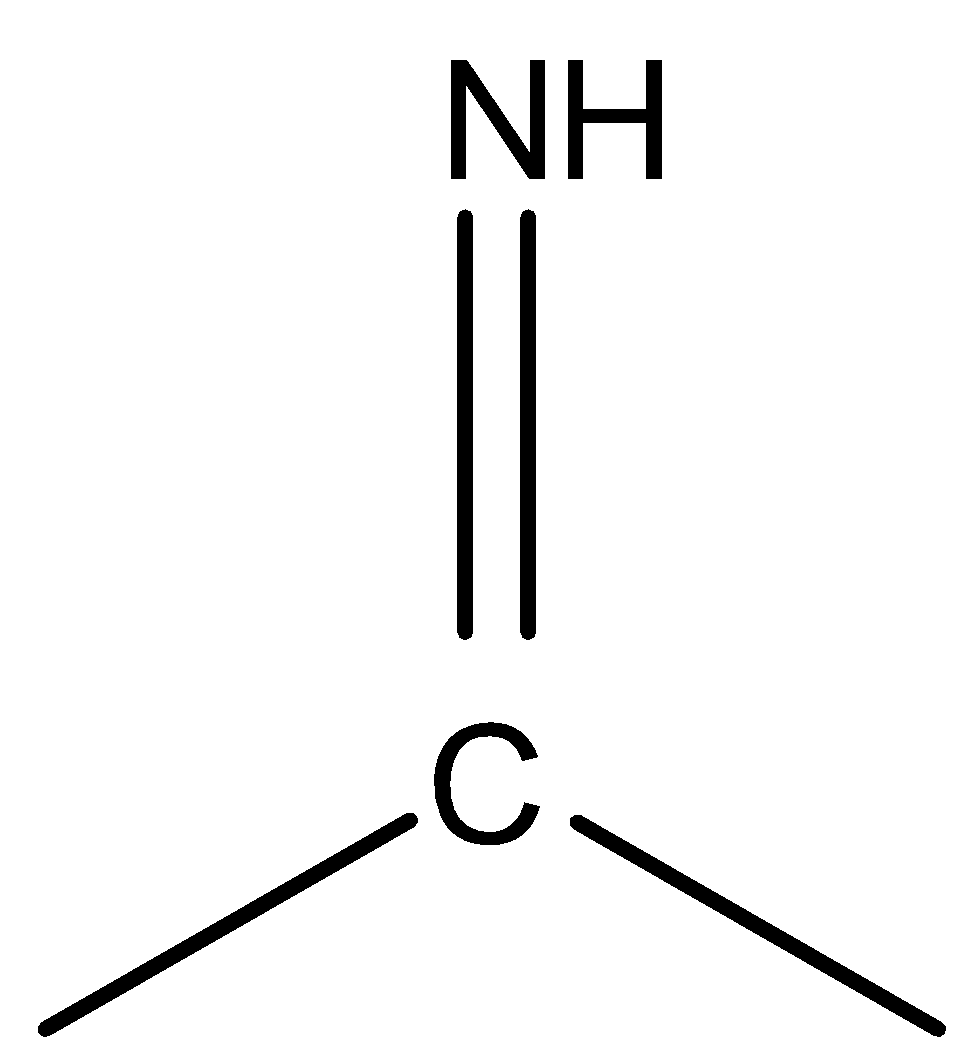 because the latter one contains a carbon- nitrogen double bond which is very stable.
because the latter one contains a carbon- nitrogen double bond which is very stable.
So, imine is more stable than enamine form. Enamine will form only when imine form formation is difficult to form in those circumstances.
So, the correct answer is Option B.
Note: Tautomerism is a type of isomerism only as there is no change in the main carbon skeleton only migration of proton or group is there, more precisely tautomerism is a functional group isomerism.
It is compulsory for the tautomer to have alpha-hydrogen for the migration or shifting. As we know that double bonds are stronger than the single bond and if the bond is between carbon and electronegative group then it is fairly more stable.
Complete step by step answer:
The above tautomerism is a functional group isomerism. In the functional group isomerism, the carbon skeleton of that compound is totally unchanged but it shows proton transfer in an intramolecular way. Since the functional group is the amine group so the tautomerism is imine-enamine isomerism.
Amines show tautomerism by getting converted to imine form. To show isomerism or tautomerism it should contain at least \[1\] alpha-hydrogen in it. This alpha-hydrogen is shifted or migrated to the nitrogen.

Enamine Imine
For stability among these two we will see the analogy between ketones and amines. In ketones the




So, imine is more stable than enamine form. Enamine will form only when imine form formation is difficult to form in those circumstances.
So, the correct answer is Option B.
Note: Tautomerism is a type of isomerism only as there is no change in the main carbon skeleton only migration of proton or group is there, more precisely tautomerism is a functional group isomerism.
It is compulsory for the tautomer to have alpha-hydrogen for the migration or shifting. As we know that double bonds are stronger than the single bond and if the bond is between carbon and electronegative group then it is fairly more stable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




