
Which of them is correct for X = [$Cr{{(N{{H}_{3}})}_{3}}C{{l}_{3}}$] and Y = [$Cr{{(gly)}_{3}}$].
(A) X shows geometrical isomerism but Y does not show geometrical isomerism.
(B) X shows optical isomerism but Y does not show optical isomerism.
(C) Y shows geometric isomerism but X does not show optical isomerism.
(D) Y shows optical isomerism but X does not show optical isomerism.
Answer
573.6k+ views
Hint: It is evident that the above mentioned compounds are complex compounds. Analyzing whether the complex X and Y are optically active and optically inactive, this may help to figure out if the compound shows optical isomerism or not.
Complete answer:
An optically active compound is those compounds which have a chiral center and it has a center of symmetry.
The coordinate compounds that can rotate when kept in front of polarised light. If it rotates the plane polarised in clockwise direction then it is said to be dextro and if rotated anticlockwise direction is known as laevo. Some compounds which are symmetrical and can rotate the plane light in the opposite direction form two isomers.
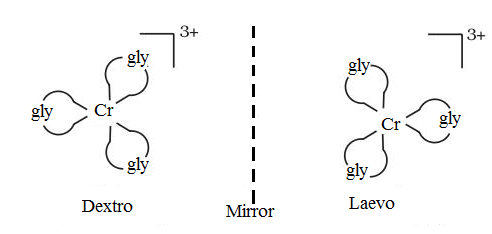
Let's see this is the case in [ $Cr{{(gly)}_{3}}$],
Complexes of the type $[M({{A}_{2}})X{{Y}_{2}}]$, $[M{{A}_{3}}]$contains a symmetrical bidentate ligand thus shows optical isomerism, From this we can conclude that [$Cr{{(gly)}_{3}}$] shows optical isomerism.
The optical isomers of [$Cr{{(gly)}_{3}}$] are as follows:
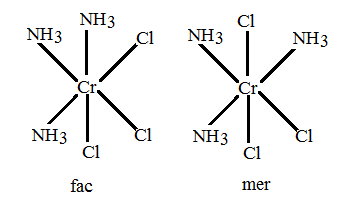
The complexes of type [$M{{A}_{3}}{{B}_{3}}$ ] show geometrical isomerism which is also known as fac-mer isomerism. So, [$Cr{{(N{{H}_{3}})}_{3}}C{{l}_{3}}$] shows geometrical isomerism. The geometrical isomers of [$Cr{{(N{{H}_{3}})}_{3}}C{{l}_{3}}$] are:
Hence, from the above discussion we can say that option A is the correct answer.
Note: In most of the cases the complexes of particular type will have the same properties as their structure. So, it is better to remember the isomerism shown by the types of complexes. This will help us answer this question within no time which is very important from an exam point of view.
Complete answer:
An optically active compound is those compounds which have a chiral center and it has a center of symmetry.
The coordinate compounds that can rotate when kept in front of polarised light. If it rotates the plane polarised in clockwise direction then it is said to be dextro and if rotated anticlockwise direction is known as laevo. Some compounds which are symmetrical and can rotate the plane light in the opposite direction form two isomers.
Let's see this is the case in [ $Cr{{(gly)}_{3}}$],
Complexes of the type $[M({{A}_{2}})X{{Y}_{2}}]$, $[M{{A}_{3}}]$contains a symmetrical bidentate ligand thus shows optical isomerism, From this we can conclude that [$Cr{{(gly)}_{3}}$] shows optical isomerism.
The optical isomers of [$Cr{{(gly)}_{3}}$] are as follows:

The complexes of type [$M{{A}_{3}}{{B}_{3}}$ ] show geometrical isomerism which is also known as fac-mer isomerism. So, [$Cr{{(N{{H}_{3}})}_{3}}C{{l}_{3}}$] shows geometrical isomerism. The geometrical isomers of [$Cr{{(N{{H}_{3}})}_{3}}C{{l}_{3}}$] are:

Hence, from the above discussion we can say that option A is the correct answer.
Note: In most of the cases the complexes of particular type will have the same properties as their structure. So, it is better to remember the isomerism shown by the types of complexes. This will help us answer this question within no time which is very important from an exam point of view.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




