
Which of the following will show optical isomerism
${\text{1 - }}$bromobutane or ${\text{2 - }}$bromobutane?
Answer
559.8k+ views
Hint: The optical isomers are the compounds that have the same number of atoms or groups present but have a different spatial arrangement of atoms or groups than each other. One can analyze the structures given and look for a chiral carbon present in the structure which gives the optical isomerism.
Complete step by step answer:
1) First of all, we will try to understand the concept of a chiral carbon. A chiral carbon is a carbon atom that is attached to the four different atoms or groups or molecules. The optical activity can only be shown by structures that have chiral carbon present in them.
2) Now let's discuss the concept of optical isomer and optical isomerism. The optical isomers are the compounds which have the same number of atoms or groups present in it but they have the different spatial arrangement of atoms or groups and this concept is called optical isomerism.
3) Now let's analyze the given structures and see for the compound which can give optical isomers. The structures are drawn below,
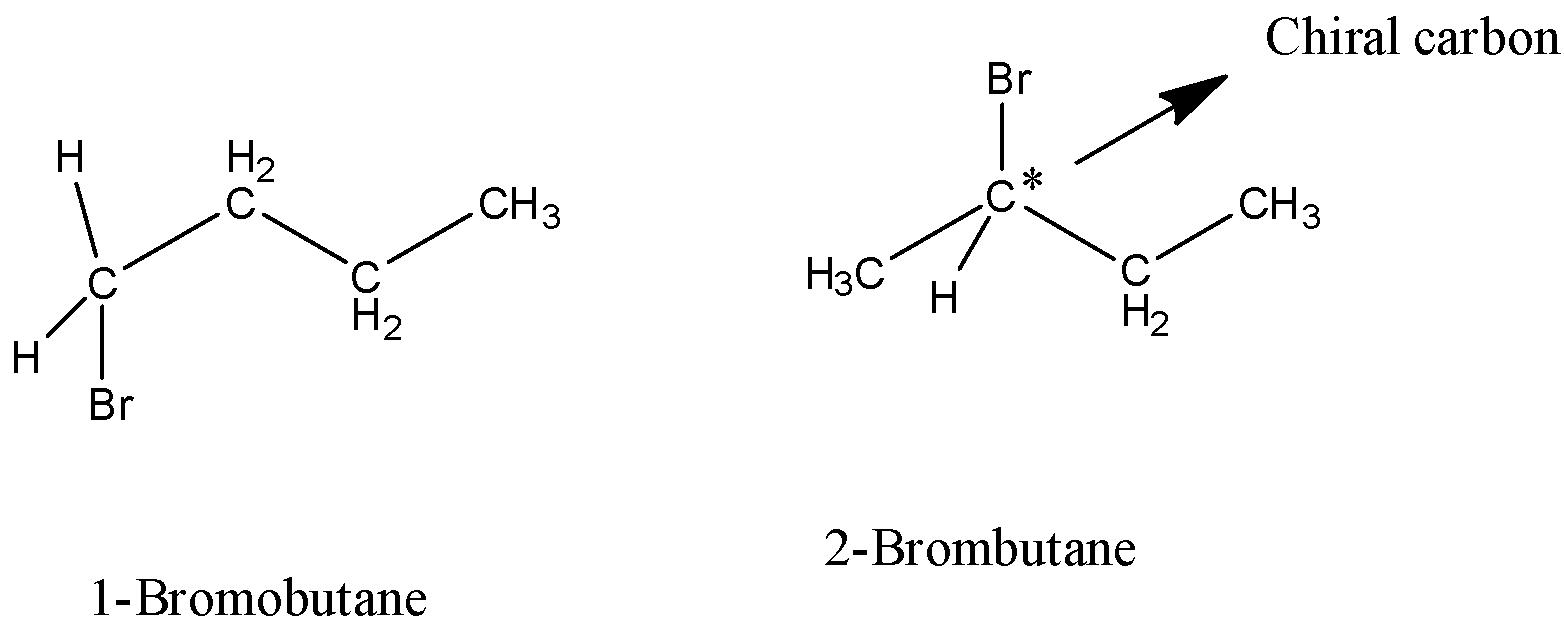
4) In the structure of ${\text{1 - }}$bromobutane there is no chiral carbon present as the carbon atom attached to the bromine atoms has the same hydrogen atoms attached to it. Hence, as it fails to fulfill the criteria of the chiral carbon, the structure cannot give optical isomers.
5) In the structure of ${\text{2 - }}$bromobutane there is a chiral carbon atom which is shown by the star mark in the structure. The carbon atom is attached to four different atoms and groups attached to it hence it is chiral carbon and it will show optical isomerism.
Note:
The optical isomers are non-superimposable mirror images of each other and they can also be called enantiomers of each other. The optical isomers of a compound can rotate the plane-polarized light towards the right or left.
Complete step by step answer:
1) First of all, we will try to understand the concept of a chiral carbon. A chiral carbon is a carbon atom that is attached to the four different atoms or groups or molecules. The optical activity can only be shown by structures that have chiral carbon present in them.
2) Now let's discuss the concept of optical isomer and optical isomerism. The optical isomers are the compounds which have the same number of atoms or groups present in it but they have the different spatial arrangement of atoms or groups and this concept is called optical isomerism.
3) Now let's analyze the given structures and see for the compound which can give optical isomers. The structures are drawn below,

4) In the structure of ${\text{1 - }}$bromobutane there is no chiral carbon present as the carbon atom attached to the bromine atoms has the same hydrogen atoms attached to it. Hence, as it fails to fulfill the criteria of the chiral carbon, the structure cannot give optical isomers.
5) In the structure of ${\text{2 - }}$bromobutane there is a chiral carbon atom which is shown by the star mark in the structure. The carbon atom is attached to four different atoms and groups attached to it hence it is chiral carbon and it will show optical isomerism.
Note:
The optical isomers are non-superimposable mirror images of each other and they can also be called enantiomers of each other. The optical isomers of a compound can rotate the plane-polarized light towards the right or left.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




