
Which of the following name reactions is not used for introducing a \[COOH\] group?
(A) Cannizzaro reaction
(B) Benzilic acid rearrangement
(C) Baeyer- Villiger oxidation
(D) Iodoform reaction
Answer
583.2k+ views
Hint: In the question, four name reactions have been mentioned and we need to choose the reaction which doesn’t introduce a carboxylic acid functional group in the product. Write the general reaction for each of the name reactions and then identify the reaction which does not introduce carboxylic acid in the product.
Complete step by step answer:
- Let’s begin by writing the general reactions of all the name reactions given in the options.
- Cannizzaro reaction is a reaction in which two molecules of carbonyl compounds having absence of $\alpha $-hydrogen atoms undergo disproportionation reaction in the presence of a base and on acid hydrolysis, form an alcohol and a carboxylic acid. One of the examples of this reaction is,
\[2HCHO\xrightarrow[{{H}_{3}}{{O}^{+}}]{NaOH}C{{H}_{2}}OH+HCOOH\]
- Benzilic acid rearrangement reaction involves conversion of a 1,2-diketone into $\alpha $-hydroxycarboxylic acid in the presence of a strong base followed by acid hydrolysis.
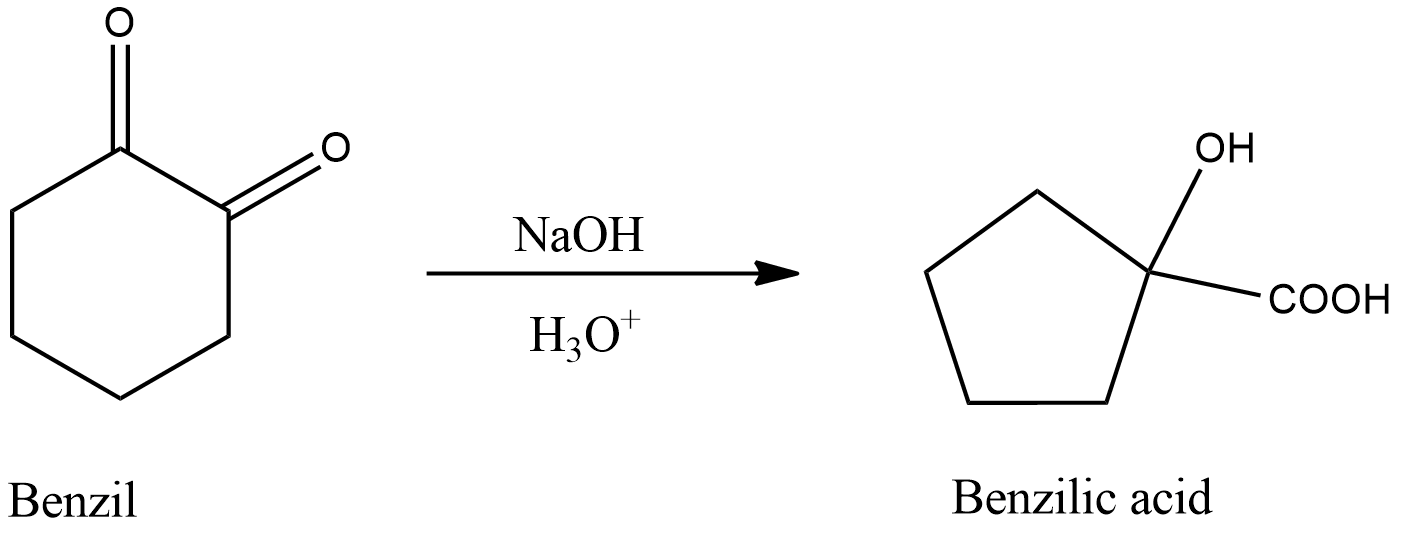
- Baeyer- Villiger oxidation is an oxidation reaction in which a ketone is converted into an ester due to addition of one oxygen atom in the chain. Here, m-chloroperoxybenzoic acid is used as an oxidizing agent.
\[C{{H}_{3}}-CO-C{{H}_{3}}\xrightarrow{m-CPBA}C{{H}_{3}}COOC{{H}_{3}}\]
- Iodoform reaction is a reaction in which a compound containing acetyl group is oxidized into salt of acetic acid due to the action of iodine in basic condition. The salt can be acidified to form carboxylic acid.
\[C{{H}_{3}}CHO\xrightarrow[(ii)HCl]{(i){{I}_{2}}/NaOH}C{{H}_{3}}COOH\]
- Therefore, Baeyer-Villiger rearrangement reaction is the only name reaction in the given options which doesn’t form carboxylic acid. So, the correct answer is “Option C”.
Note: Remember the important name reactions in organic chemistry. Remember to convert ketone to ester, Baeyer-Villiger rearrangement reaction is used. Iodoform reaction will be given by compounds containing methoxy ketone group and ethanol and isopropanol or sec-alcohols which form methoxy ketones on oxidation.
Complete step by step answer:
- Let’s begin by writing the general reactions of all the name reactions given in the options.
- Cannizzaro reaction is a reaction in which two molecules of carbonyl compounds having absence of $\alpha $-hydrogen atoms undergo disproportionation reaction in the presence of a base and on acid hydrolysis, form an alcohol and a carboxylic acid. One of the examples of this reaction is,
\[2HCHO\xrightarrow[{{H}_{3}}{{O}^{+}}]{NaOH}C{{H}_{2}}OH+HCOOH\]
- Benzilic acid rearrangement reaction involves conversion of a 1,2-diketone into $\alpha $-hydroxycarboxylic acid in the presence of a strong base followed by acid hydrolysis.

- Baeyer- Villiger oxidation is an oxidation reaction in which a ketone is converted into an ester due to addition of one oxygen atom in the chain. Here, m-chloroperoxybenzoic acid is used as an oxidizing agent.
\[C{{H}_{3}}-CO-C{{H}_{3}}\xrightarrow{m-CPBA}C{{H}_{3}}COOC{{H}_{3}}\]
- Iodoform reaction is a reaction in which a compound containing acetyl group is oxidized into salt of acetic acid due to the action of iodine in basic condition. The salt can be acidified to form carboxylic acid.
\[C{{H}_{3}}CHO\xrightarrow[(ii)HCl]{(i){{I}_{2}}/NaOH}C{{H}_{3}}COOH\]
- Therefore, Baeyer-Villiger rearrangement reaction is the only name reaction in the given options which doesn’t form carboxylic acid. So, the correct answer is “Option C”.
Note: Remember the important name reactions in organic chemistry. Remember to convert ketone to ester, Baeyer-Villiger rearrangement reaction is used. Iodoform reaction will be given by compounds containing methoxy ketone group and ethanol and isopropanol or sec-alcohols which form methoxy ketones on oxidation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




