
Which of the following ligands is tetradentate?
A.
B.
C.
D.




Answer
573.3k+ views
Hint: In the coordination chemistry, the classification of ligands is done on the basis of the number of sites to which the ligand is binded with the central metal atom to form the coordination complex or coordination ion.
Complete step by step answer:
Ligands are defined as an ion or a molecule which releases its electron pair to the central metal atom or ion to create a coordination complex. Ligands used in forming the complex can be anion, cation or neutral molecule.
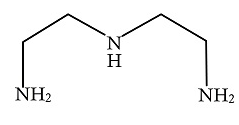
A.
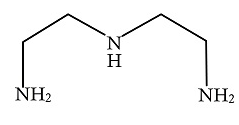
In the above given compound, three lone pairs are present in three nitrogen atoms, hence there are three donor atoms present which donates its lone pair of electrons to the central metal atom. Therefore, it is a tridentate ligand.
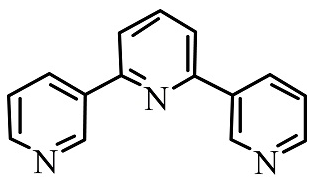
B.
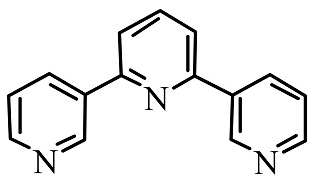
In the above given compound, three lone pairs are present in three nitrogen atoms, hence there are three donor atoms present which donates its lone pair of electrons to the central metal atom. Therefore, it is a tridentate ligand.
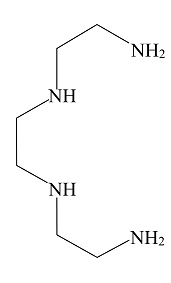
C.
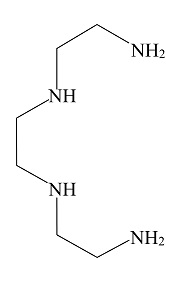
In the above given compound, four lone pairs are present in four nitrogen atoms, hence there are four donor atoms present which donates its lone pair of electrons to the central metal atom. Therefore, it is a tetradentate ligand.
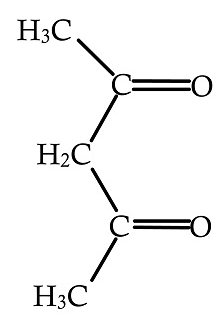
D.
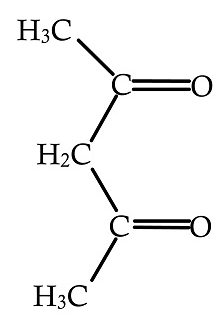
In the above given compound, two lone pairs are present in two oxygen atoms, hence there are two donor atoms present which donates its lone pair of electrons to the central metal atom. Therefore, it is bidentate ligand.
So, the correct answer is Option C.
Note: In the coordination complex, the ligand is the one which behaves as an lewis base. Lewis bases are those which donate pairs of electrons. The central metal atom behaves as lewis acid. Lewis acid are those who accept the pair of electrons.
Complete step by step answer:
Ligands are defined as an ion or a molecule which releases its electron pair to the central metal atom or ion to create a coordination complex. Ligands used in forming the complex can be anion, cation or neutral molecule.
A.

In the above given compound, three lone pairs are present in three nitrogen atoms, hence there are three donor atoms present which donates its lone pair of electrons to the central metal atom. Therefore, it is a tridentate ligand.
B.

In the above given compound, three lone pairs are present in three nitrogen atoms, hence there are three donor atoms present which donates its lone pair of electrons to the central metal atom. Therefore, it is a tridentate ligand.
C.

In the above given compound, four lone pairs are present in four nitrogen atoms, hence there are four donor atoms present which donates its lone pair of electrons to the central metal atom. Therefore, it is a tetradentate ligand.
D.

In the above given compound, two lone pairs are present in two oxygen atoms, hence there are two donor atoms present which donates its lone pair of electrons to the central metal atom. Therefore, it is bidentate ligand.
So, the correct answer is Option C.
Note: In the coordination complex, the ligand is the one which behaves as an lewis base. Lewis bases are those which donate pairs of electrons. The central metal atom behaves as lewis acid. Lewis acid are those who accept the pair of electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




