
Which of the following is not cleaved by HI even at 525K?
A.${{C}_{6}}{{H}_{5}}-O-C{{H}_{3}}$
B.${{C}_{6}}{{H}_{5}}O{{C}_{3}}{{H}_{7}}$
C.
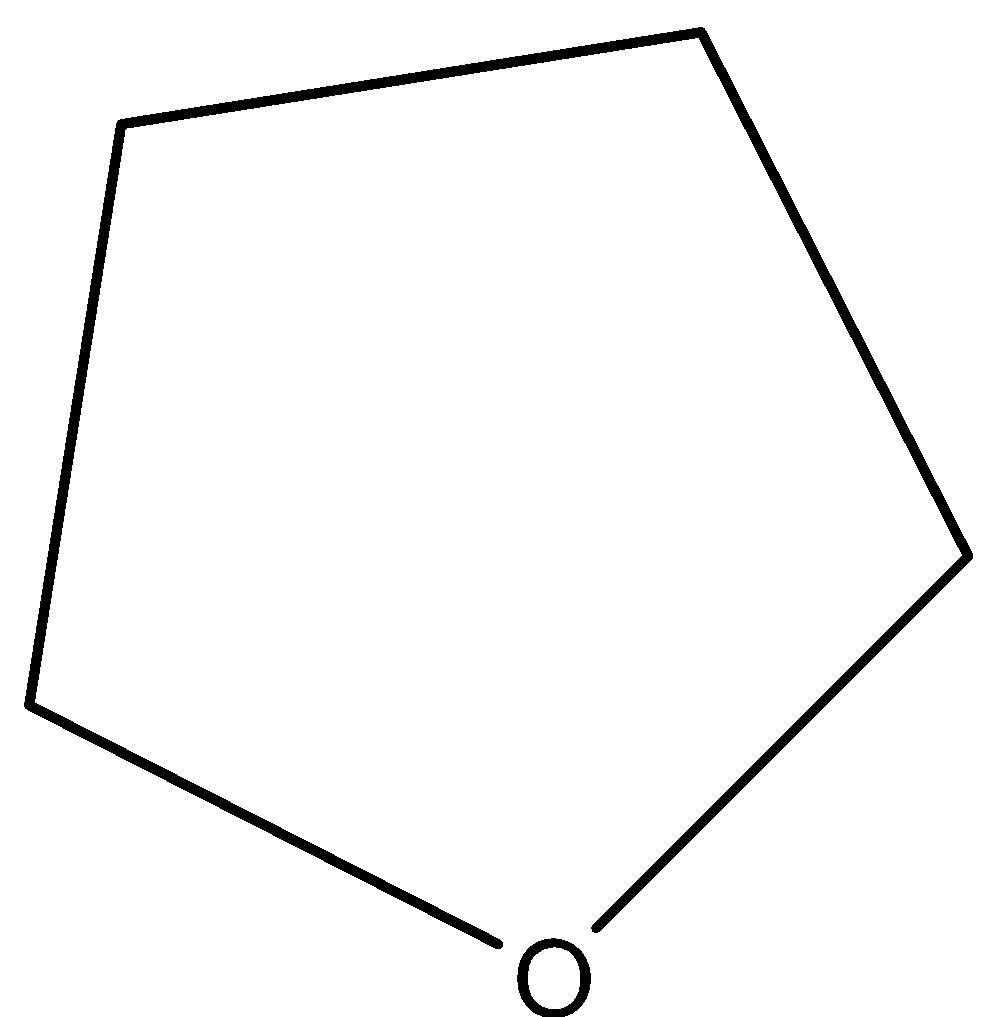
D.${{C}_{6}}{{H}_{5}}-O-{{C}_{6}}{{H}_{5}}$

Answer
521.7k+ views
Hint: In cleavage reaction, the -OR group is replaced by -OH group, in case of alkyl aryl ethers. Reaction of ether with concentrated HI starts with the protonation of the ether, and iodide is a good nucleophile.
Complete step by step solution:
In order to answer our question, we need to learn properties about ethers, and cleavage reactions. Ethers can be classified as symmetrical or simple, if the aryl or alkyl group that is attached to the atom of oxygen are the same, and unsymmetrical or mixed, if the two groups are not the same. The nomenclature of ethers are generally found from the names of aryl/alkyl groups that are written as different words in alphabetical order and then we add the word ether at the end. The IUPAC system of nomenclature tells that ethers are regarded as derivatives of hydrocarbons that are chosen as the parent hydrocarbon. Alkyl groups have large size, so (then H) R-0-R bond angle in ethers is large (> 110) and that is the reason why net dipole-moment becomes less. Some physical properties of ethers include:
i.Low boiling points
ii. Low solubility in water
Solubility in water further decreases with increasing molar mass of ethers as ethereal oxygen becomes more hindered and become less available to intermolecular H-bonding with water
Now, let us learn about the cleavage of bonds in ethers. Ethers due to low polarity are least reactive among other functional groups. With strong acids they form alkyl halide and alcohols by C-O bond cleavage:
\[RC{{H}_{2}}-O-C{{H}_{2}}R+HX\to RC{{H}_{2}}OH+RC{{H}_{2}}-X\]
Alkyl aryl ethers are cleaved at the alkyl-oxygen bord due to the more stable aryl oxygen bond. Let us come to our question. Now, cyclic ethers do not give us cleavage reactions with HI.
So, in our case, option C does not react with HI as a cleavage reaction and hence, it is the correct answer.
NOTE: Ether are generally very stable compounds. Due to this high chemical stability, it is very rare for cleavage reactions to take place. However, they can only take place only if there are extreme conditions, like high temperature.
Complete step by step solution:
In order to answer our question, we need to learn properties about ethers, and cleavage reactions. Ethers can be classified as symmetrical or simple, if the aryl or alkyl group that is attached to the atom of oxygen are the same, and unsymmetrical or mixed, if the two groups are not the same. The nomenclature of ethers are generally found from the names of aryl/alkyl groups that are written as different words in alphabetical order and then we add the word ether at the end. The IUPAC system of nomenclature tells that ethers are regarded as derivatives of hydrocarbons that are chosen as the parent hydrocarbon. Alkyl groups have large size, so (then H) R-0-R bond angle in ethers is large (> 110) and that is the reason why net dipole-moment becomes less. Some physical properties of ethers include:
i.Low boiling points
ii. Low solubility in water
Solubility in water further decreases with increasing molar mass of ethers as ethereal oxygen becomes more hindered and become less available to intermolecular H-bonding with water
Now, let us learn about the cleavage of bonds in ethers. Ethers due to low polarity are least reactive among other functional groups. With strong acids they form alkyl halide and alcohols by C-O bond cleavage:
\[RC{{H}_{2}}-O-C{{H}_{2}}R+HX\to RC{{H}_{2}}OH+RC{{H}_{2}}-X\]
Alkyl aryl ethers are cleaved at the alkyl-oxygen bord due to the more stable aryl oxygen bond. Let us come to our question. Now, cyclic ethers do not give us cleavage reactions with HI.
So, in our case, option C does not react with HI as a cleavage reaction and hence, it is the correct answer.
NOTE: Ether are generally very stable compounds. Due to this high chemical stability, it is very rare for cleavage reactions to take place. However, they can only take place only if there are extreme conditions, like high temperature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




