
Which of the following is most basic?
A. Diphenylamine
B. Triphenylamine
C. p-Nitroaniline
D. Benzylamine
Answer
533.7k+ views
Hint: Initially our question is talking about the basic characteristics. Basicity of any base is the property of that compound of replacing the number of hydrogens from itself in presence of an acid. Mostly it is affected by many factors like Effect of Charge, Resonance Stability, Inductive effect, Mesomeric effect and many more.
Complete step by step answer:
Firstly, we should check which effect is working the best on the basic property. You can easily notice it is all about the\[\;N{H_2}\] group. The \[\;N{H_2}\] group is the conjugate of Ammonia. Since ammonia is a weak base therefore its conjugate base must be a strong base.
Here Inductive effect is working at prior stage.
\[C_6H_5 - CH_2 - NH_2\] popularly known as Benzylamine is more basic in all of the above mentioned because benzyl group is an electron donating group just because of +I effect. So, it is able to increase electron density of N of \[ - N{H_2}\] group.
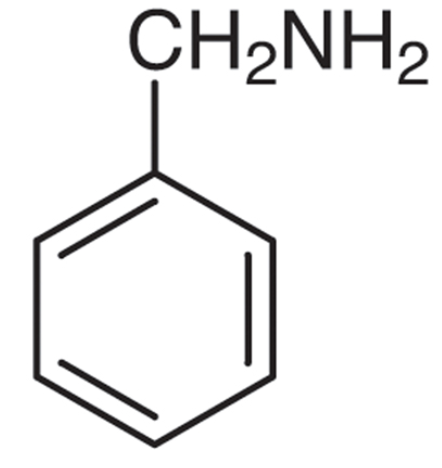
Thus, due to higher electron density, the rate of donation of a free pair of electrons is increased. Since the electron density is higher this means its basic property is higher. While phenyl and nitro group are electron withdrawing group so they are able to decrease the electron density of N of \[ - N{H_2}\] group. Hence, they are less basic.
So, the correct answer is Option d.
Note: The inductive effect increases and decreases the electron density in the molecule. The +I group increases the electron density in the complete molecule which makes it easy for the compound to donate electrons easily. Whereas -I effect decreases the electron density making it electron deficient. Thus, we can say +I group helps in making compound basic while -I group makes it acidic.
In the gaseous phase the basic order of amine is \[3^\circ > 2^\circ > 1^\circ > NH3\].
Complete step by step answer:
Firstly, we should check which effect is working the best on the basic property. You can easily notice it is all about the\[\;N{H_2}\] group. The \[\;N{H_2}\] group is the conjugate of Ammonia. Since ammonia is a weak base therefore its conjugate base must be a strong base.
Here Inductive effect is working at prior stage.
\[C_6H_5 - CH_2 - NH_2\] popularly known as Benzylamine is more basic in all of the above mentioned because benzyl group is an electron donating group just because of +I effect. So, it is able to increase electron density of N of \[ - N{H_2}\] group.

Thus, due to higher electron density, the rate of donation of a free pair of electrons is increased. Since the electron density is higher this means its basic property is higher. While phenyl and nitro group are electron withdrawing group so they are able to decrease the electron density of N of \[ - N{H_2}\] group. Hence, they are less basic.
So, the correct answer is Option d.
Note: The inductive effect increases and decreases the electron density in the molecule. The +I group increases the electron density in the complete molecule which makes it easy for the compound to donate electrons easily. Whereas -I effect decreases the electron density making it electron deficient. Thus, we can say +I group helps in making compound basic while -I group makes it acidic.
In the gaseous phase the basic order of amine is \[3^\circ > 2^\circ > 1^\circ > NH3\].
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




