
Which of the following is aryl alkyl halide?
(A) o-chlorotoluene
(B) o-bromochlorobenzene
(C) 1-chloro-2-phenylethane
(D) Toluene
Answer
233.1k+ views
Hint: In Aryl alkyl halides, aryl stands for aromatic compound, alkyl stands for any compound having carbon and hydrogen atoms only and halide stands for halogen family members such as fluorine, chlorine, bromine, etc.
Complete step-by-step solution:
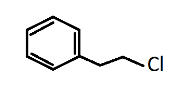
Alkyl aryl halide is formed when one or more hydrogen atoms in an aromatic alkane are replaced by halogen atoms like fluorine, chlorine, bromine or iodine. This can be seen in option (C).
1-chloro-2-phenylethane contains benzene as an aryl group and chloroethane as an alkyl halide with ethane being the alkyl group and chlorine being the halide substituent.
While option (D), toluene has no halogen in it, so it can’t be an aryl alkyl halide as it has only methyl groups on benzene rings. But if one of the hydrogens of methane is replaced by any halogen, it will become aryl alkyl halide.
Similarly, we see in option (B), two halogens are present as substituents but no alkyl group is attached to the benzene ring. So, it is just an aryl halide.
Hence, the correct option is (C).
Note: Option (A) also accounts for the same because it has a chlorine at ortho position of benzene ring and toluene, methyl group on the benzene ring. This might look as an alkyl aryl halide, but it is just an aryl halide, as the halide is bonded directly to the benzene ring in it.
Complete step-by-step solution:
Alkyl aryl halide is formed when one or more hydrogen atoms in an aromatic alkane are replaced by halogen atoms like fluorine, chlorine, bromine or iodine. This can be seen in option (C).
1-chloro-2-phenylethane contains benzene as an aryl group and chloroethane as an alkyl halide with ethane being the alkyl group and chlorine being the halide substituent.

While option (D), toluene has no halogen in it, so it can’t be an aryl alkyl halide as it has only methyl groups on benzene rings. But if one of the hydrogens of methane is replaced by any halogen, it will become aryl alkyl halide.
Similarly, we see in option (B), two halogens are present as substituents but no alkyl group is attached to the benzene ring. So, it is just an aryl halide.
Hence, the correct option is (C).
Note: Option (A) also accounts for the same because it has a chlorine at ortho position of benzene ring and toluene, methyl group on the benzene ring. This might look as an alkyl aryl halide, but it is just an aryl halide, as the halide is bonded directly to the benzene ring in it.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




