
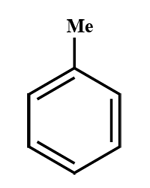
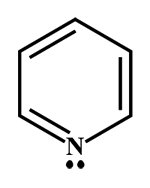
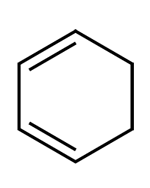
Which of the following does not react with aqueous\[KMn{O_4}\]?
A.
B. \[{F_2}C = C{F_2}\]
C.
D.
In this question more than one option is correct.



Answer
601.2k+ views
Hint: The first thing after reading this question comes to our mind is Bromine Water Test. We all know that bromine has a blue colour. As in double bonded compounds bromine is used up. Therefore, its colour will be gone and the solution in which it was added will become colourless. But when there are only single bonded structures the colour will persist. Also, we will learn about unsaturation, \[KMn{O_4}\] test (Baeyer’s test).
Complete answer:
Unsaturation- Unsaturation refers to the degree of double bond present in a substance. Degrees of unsaturation can also be named as index of hydrogen deficiency (IHD), double bond equivalents, or unsaturation index. It is a method which is also used to find rings and double bonds. It helps us to make chemical structures.
Baeyer’s test – In this test we will take an organic compound and after dissolving it we will add alkaline potassium permanganate drop by drop. As potassium permanganate is pink in colour, therefore if pink colour remains that there will be a saturated compound. If it disappears then there will be a double bonded structure.
A and C are aromatic (delocalisation \[\pi \] \[{e^ - }\]will occur, that is bromine water test will give a positive result.
B. Due to EWG, four \[F\] atoms, electron density or nucleophilicity around (C=C) decreases and the compound does not give a test for unsaturation.
D. It is an unsaturated compound and gives the test for unsaturation.
So, as we can see, options (A, B, C) do not react with aqueous $KMn{O_4}$.
So, the correct answer is “Option A, B, C”.
Note: Bromine Water Test is a qualitative test used to find if there is a double bond or not. In this test we find the presence and absence of unsaturation. Bromine being a halogen atom will be going to attack the \[\pi \] electrons present in alkanes and alkynes. This means that bromine shows no colour difference in single bonded structures, but there is a change when double bond is present.
Complete answer:
Unsaturation- Unsaturation refers to the degree of double bond present in a substance. Degrees of unsaturation can also be named as index of hydrogen deficiency (IHD), double bond equivalents, or unsaturation index. It is a method which is also used to find rings and double bonds. It helps us to make chemical structures.
Baeyer’s test – In this test we will take an organic compound and after dissolving it we will add alkaline potassium permanganate drop by drop. As potassium permanganate is pink in colour, therefore if pink colour remains that there will be a saturated compound. If it disappears then there will be a double bonded structure.
A and C are aromatic (delocalisation \[\pi \] \[{e^ - }\]will occur, that is bromine water test will give a positive result.
B. Due to EWG, four \[F\] atoms, electron density or nucleophilicity around (C=C) decreases and the compound does not give a test for unsaturation.
D. It is an unsaturated compound and gives the test for unsaturation.
So, as we can see, options (A, B, C) do not react with aqueous $KMn{O_4}$.
So, the correct answer is “Option A, B, C”.
Note: Bromine Water Test is a qualitative test used to find if there is a double bond or not. In this test we find the presence and absence of unsaturation. Bromine being a halogen atom will be going to attack the \[\pi \] electrons present in alkanes and alkynes. This means that bromine shows no colour difference in single bonded structures, but there is a change when double bond is present.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




