
Which of the following describes the given graph correctly?
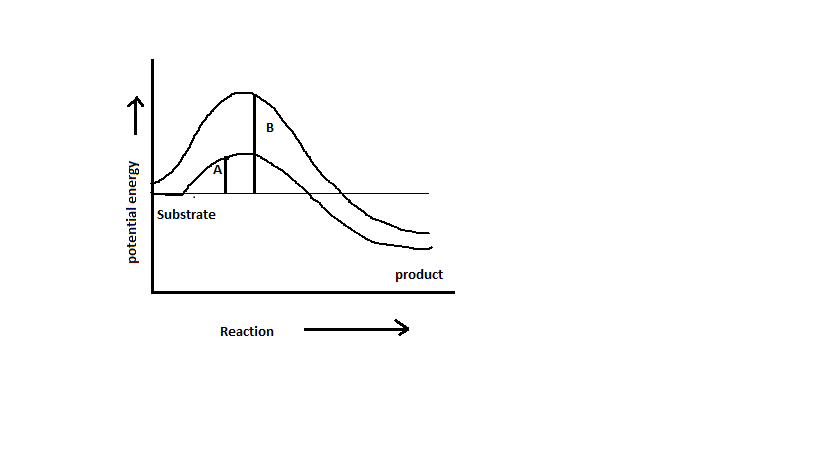
A. Endothermic reaction with energy A in absence of enzyme and B in presence of enzyme.
B. Exothermic reaction with energy A in absence of enzyme and B in presence of enzyme.
C. Exothermic reaction with energy A in presence of enzyme and B in absence of enzyme.
D. Endothermic reaction with energy A in presence of enzyme and B in absence of enzyme.

Answer
573.6k+ views
Hint:-Endothermic reaction needs energy for the reaction. This type of reaction substrate is at a higher energy level than products. Activation energy is required to form products. In the presence of enzymes, the rate of reaction increases and becomes stable. In the absence of enzymes, activation energy increases.
Complete Answer:-
The conversion of reactants into products is called a chemical reaction. Chemical reactions need enzymes or catalysts to increase the rate of reaction. High energy is required for the formation of transition state, alignment of reactant groups, formation of transient unstable charges, rearrangement of chemical bonds, etc. This type of energy required to perform chemical reactions is called activation energy. The activation energy of the transition state can be lowered by enzymes and hence increasing the rate of reaction.
The reactant absorbs heat energy from the surrounding to form products in the endothermic reactions. Products are at higher energy levels than reactants in endothermic reactions.
Energy is released in the exothermic reaction in the form of heat and light. The product’s total energy is less than the reactant’s total energy.
In the given graph, the energy of the substrate is higher than that of the product. It shows the endothermic nature of the reaction. A and B shows the activation energy levels. In the presence of enzymes the activation energy is lowered from B to A.
Thus, the right option is C.
Note:- Endothermic reactions give a cooling effect on the environment by lowering the temperature from the surrounding. Melting of ice cubes, solid salts, sublimation of dry ice into carbon dioxide gas, converting frost to water vapor, etc are examples of endothermic reactions. During this process if we heat the reactants and it cools down during the reaction then it is said to be an endothermic reaction.
Complete Answer:-
The conversion of reactants into products is called a chemical reaction. Chemical reactions need enzymes or catalysts to increase the rate of reaction. High energy is required for the formation of transition state, alignment of reactant groups, formation of transient unstable charges, rearrangement of chemical bonds, etc. This type of energy required to perform chemical reactions is called activation energy. The activation energy of the transition state can be lowered by enzymes and hence increasing the rate of reaction.
The reactant absorbs heat energy from the surrounding to form products in the endothermic reactions. Products are at higher energy levels than reactants in endothermic reactions.
Energy is released in the exothermic reaction in the form of heat and light. The product’s total energy is less than the reactant’s total energy.
In the given graph, the energy of the substrate is higher than that of the product. It shows the endothermic nature of the reaction. A and B shows the activation energy levels. In the presence of enzymes the activation energy is lowered from B to A.
Thus, the right option is C.
Note:- Endothermic reactions give a cooling effect on the environment by lowering the temperature from the surrounding. Melting of ice cubes, solid salts, sublimation of dry ice into carbon dioxide gas, converting frost to water vapor, etc are examples of endothermic reactions. During this process if we heat the reactants and it cools down during the reaction then it is said to be an endothermic reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




