
Which of the following contains a coordinate covalent bond?
A.Water
B.Ammonia
C.Ammonium ion
D.Ethylene
Answer
517.5k+ views
Hint: The type of bonding in which both shared electrons are donated by the same atom is known as coordinate bonding. It is also known as dative bonding and it is a directional bond i.e., sharing of electrons takes place in a definite direction.
Complete answer:
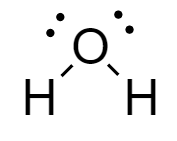
Water: In the molecule, an oxygen atom is covalently bonded to two hydrogen atoms forming a bent shape structure. The structure of water molecule is as follows:
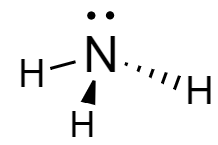
Ammonia: In the molecule, the nitrogen atom is covalently bonded to the three hydrogen atoms forming a tetrahedral structure. The structure of ammonia is drawn as follows:
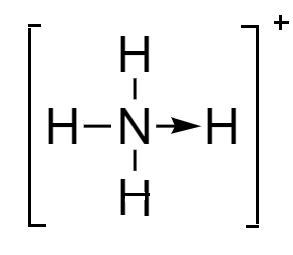
Ammonium ion: In this ion, the fourth hydrogen atom is attached to the nitrogen atom via coordinate bond because the valency of nitrogen is completed but it still has a lone pair to donate to the hydrogen nucleus. Although the representation of bonds is done differently, they are almost similar to the covalent bonds. The structure of ammonium ion is as follows:
Ethylene: It is an unsaturated hydrocarbon consisting of two carbon atoms covalently bonded to each via triple bond and two hydrogen atoms are attached via single bond. The structure of ethylene is represented as follows:
\(HC \equiv CH\)
Hence, the compound which contains covalent coordinate bonds is ammonium ion.
Thus, option (C) is the correct answer.
Note:
Both covalent and coordinate are formed when sharing of electrons takes place between two atoms. Therefore after the formation of bond, both types of bonding seem to be identical but the key difference is that in covalent bond, mutual sharing of electrons takes place between atoms whereas in coordinate bond, one atom acts as a donor and donates both of its electrons.
Complete answer:
Water: In the molecule, an oxygen atom is covalently bonded to two hydrogen atoms forming a bent shape structure. The structure of water molecule is as follows:

Ammonia: In the molecule, the nitrogen atom is covalently bonded to the three hydrogen atoms forming a tetrahedral structure. The structure of ammonia is drawn as follows:

Ammonium ion: In this ion, the fourth hydrogen atom is attached to the nitrogen atom via coordinate bond because the valency of nitrogen is completed but it still has a lone pair to donate to the hydrogen nucleus. Although the representation of bonds is done differently, they are almost similar to the covalent bonds. The structure of ammonium ion is as follows:

Ethylene: It is an unsaturated hydrocarbon consisting of two carbon atoms covalently bonded to each via triple bond and two hydrogen atoms are attached via single bond. The structure of ethylene is represented as follows:
\(HC \equiv CH\)
Hence, the compound which contains covalent coordinate bonds is ammonium ion.
Thus, option (C) is the correct answer.
Note:
Both covalent and coordinate are formed when sharing of electrons takes place between two atoms. Therefore after the formation of bond, both types of bonding seem to be identical but the key difference is that in covalent bond, mutual sharing of electrons takes place between atoms whereas in coordinate bond, one atom acts as a donor and donates both of its electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




