
Which of the following compounds is an allylic alcohol?
(a)
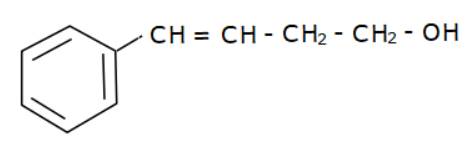
(b)
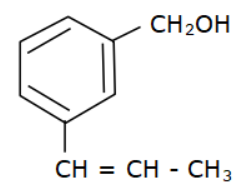
(c)
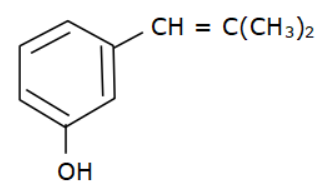
(d)
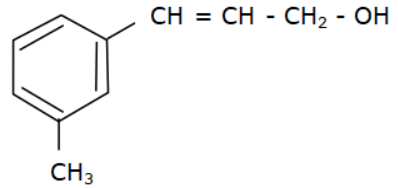
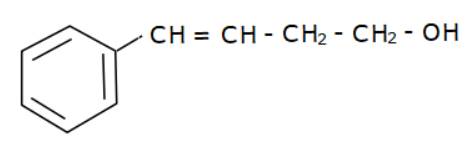



Answer
517.5k+ views
Hint: In order to attempt this question, we need to know the basic concepts of alcohol and allyl group, which are very important in Organic Chemistry. Alcohols have hydroxyl groups in their molecule, while allyl group is that group in which the carbon atom attached to the main molecule is attached to a carbon atom which is doubly bonded to another carbon atom.
Complete answer:
Alcohols are organic compounds which carry a functional group $ - OH$ , which is a hydroxyl group. Every alcohol group must contain at least one hydroxyl group which is bound to a saturated carbon atom of the compound. The simplest alcohol known is Methanol having the molecular formula $C{H_3}OH.$ Some other alcohols are Ethanol, Propanol, Butanol, Phenol et cetera. Phenol is the simplest aromatic alcohol, having the molecular formula ${C_6}{H_5}OH.$
Allyl group is a substituent group. Its formula is \[{H_2}C = CH - C{H_2} - R\] , where R is the molecule excluding the allylic group.
Allylic alcohols are organic compounds having formula \[{H_2}C = CH - C{H_2} - OH.\] A substituted allyl alcohol will have the formula \[R - CH = CH - C{H_2} - OH.\]
Options (a), (b) and (c) do not have an allyl group. Options (d) has the formula \[R - CH = CH - C{H_2} - OH\] where R is Toluene $\left( {{C_6}{H_5} - C{H_3}} \right).$
Hence, the required answer is (d)
Note:
The IUPAC name of allyl alcohol is $prop - 2 - en - ol.$ Allyl alcohols on substitution can form different allylic alcohols. The name “allyl” is derived from the Latin name of Garlic, Allium Sativum. There are groups very similar to allyl groups in Chemistry and can be mistaken for it, hence we have to be careful while attempting these types of questions.
Complete answer:
Alcohols are organic compounds which carry a functional group $ - OH$ , which is a hydroxyl group. Every alcohol group must contain at least one hydroxyl group which is bound to a saturated carbon atom of the compound. The simplest alcohol known is Methanol having the molecular formula $C{H_3}OH.$ Some other alcohols are Ethanol, Propanol, Butanol, Phenol et cetera. Phenol is the simplest aromatic alcohol, having the molecular formula ${C_6}{H_5}OH.$
Allyl group is a substituent group. Its formula is \[{H_2}C = CH - C{H_2} - R\] , where R is the molecule excluding the allylic group.
Allylic alcohols are organic compounds having formula \[{H_2}C = CH - C{H_2} - OH.\] A substituted allyl alcohol will have the formula \[R - CH = CH - C{H_2} - OH.\]
Options (a), (b) and (c) do not have an allyl group. Options (d) has the formula \[R - CH = CH - C{H_2} - OH\] where R is Toluene $\left( {{C_6}{H_5} - C{H_3}} \right).$
Hence, the required answer is (d)

Note:
The IUPAC name of allyl alcohol is $prop - 2 - en - ol.$ Allyl alcohols on substitution can form different allylic alcohols. The name “allyl” is derived from the Latin name of Garlic, Allium Sativum. There are groups very similar to allyl groups in Chemistry and can be mistaken for it, hence we have to be careful while attempting these types of questions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




