
Which of the following compounds forms cross-linked silicones upon hydrolysis followed by polymerization?
A.

B.

C.
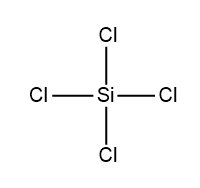
D.





Answer
517.2k+ views
Hint: When a synthetic polymer or a natural polymer links to another polymer via covalent or ionic bonds, then this type of bonding is termed as cross link bonding. In simple words, crosslinking is the process of forming chemical bonds of relatively short sequence in order to combine polymer chains together.
Complete answer:A silicone is a polymer which consists of silicon, oxygen and alkyl groups. It is formed using siloxanes and silanes which have a use in industries like medicine, adhesive etc. These are also known as high temperature polymers due to their high thermal stability.
Based on the structure of monomer taken while performing reaction, silicones can be of three types:
1.Linear silicones: These are obtained when there are two alkyl groups present on siloxanes i.e., are obtained by the hydrolysis of diary or dialkyl silicon chlorides followed by the polymerization reaction. The base unit to obtain a cross linked polymer is ${{R}_{2}}SiC{{l}_{2}}$.
2.Cyclic silicones: If in case, the water molecules are removed from the terminal points of linear silicones i.e., silicones formed using ${{R}_{2}}SiC{{l}_{2}}$, then it can be transformed into cyclic silicones or ring silicones.
3.Cross linked silicones: When the hydrolysis of monoalkylchloro silanes takes place, followed by the polymerization reaction then a very complex structure is formed which is termed as cross-linked polymer. The base unit to obtain a cross linked polymer is $RSiC{{l}_{3}}$.
Among given options, the structure of monoalkylchloro silane is given as follows:
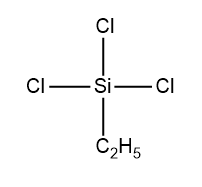
Hence, it is the only compound which can form a cross linked silicone.
Formation of cross-linked silicone using given compound takes place as follows:
Step-1: Addition of water molecule:
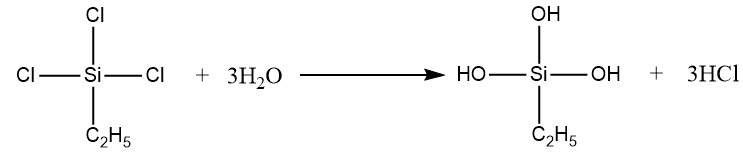
Step-2: Polymerization reaction takes place by removal of water molecules.
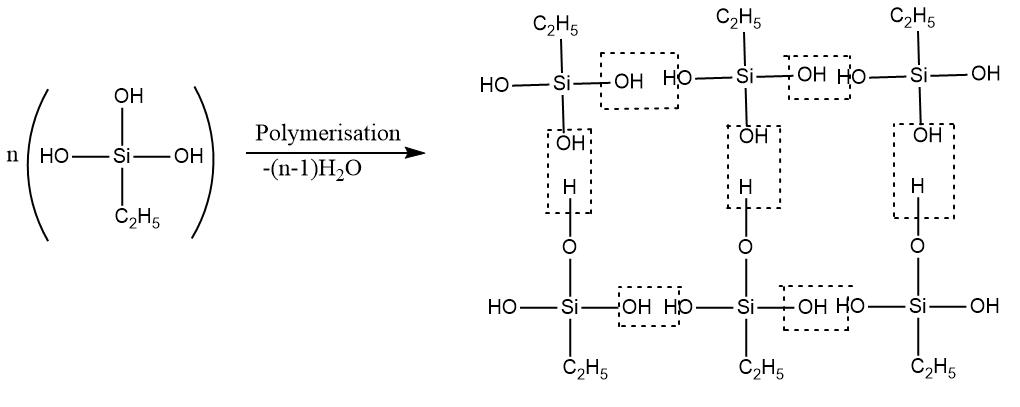
Therefore, the structure of cross-linked silicone formed is as follows:
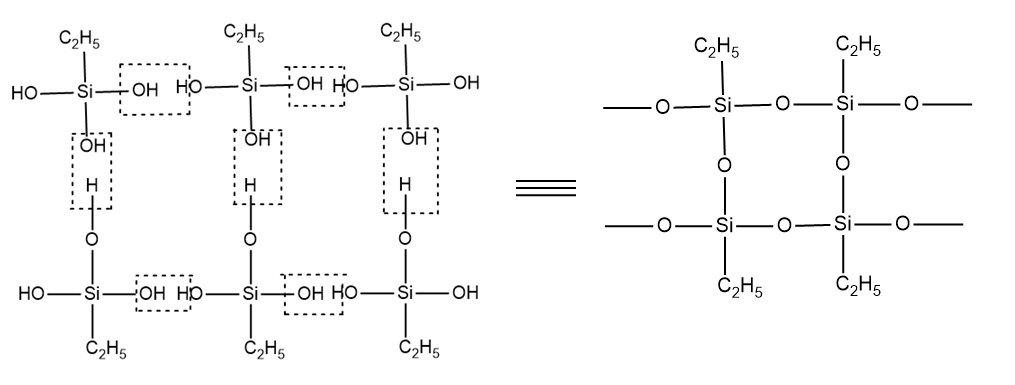
Thus, option (B) is the correct answer.
Note:
It is important to note that the nature of the alkyl group present and the extent of cross linking is responsible to determine the nature of polymer formed. The nature of polymers may vary from oily liquids to solids having rubber like properties.
Complete answer:A silicone is a polymer which consists of silicon, oxygen and alkyl groups. It is formed using siloxanes and silanes which have a use in industries like medicine, adhesive etc. These are also known as high temperature polymers due to their high thermal stability.
Based on the structure of monomer taken while performing reaction, silicones can be of three types:
1.Linear silicones: These are obtained when there are two alkyl groups present on siloxanes i.e., are obtained by the hydrolysis of diary or dialkyl silicon chlorides followed by the polymerization reaction. The base unit to obtain a cross linked polymer is ${{R}_{2}}SiC{{l}_{2}}$.
2.Cyclic silicones: If in case, the water molecules are removed from the terminal points of linear silicones i.e., silicones formed using ${{R}_{2}}SiC{{l}_{2}}$, then it can be transformed into cyclic silicones or ring silicones.
3.Cross linked silicones: When the hydrolysis of monoalkylchloro silanes takes place, followed by the polymerization reaction then a very complex structure is formed which is termed as cross-linked polymer. The base unit to obtain a cross linked polymer is $RSiC{{l}_{3}}$.
Among given options, the structure of monoalkylchloro silane is given as follows:

Hence, it is the only compound which can form a cross linked silicone.
Formation of cross-linked silicone using given compound takes place as follows:
Step-1: Addition of water molecule:

Step-2: Polymerization reaction takes place by removal of water molecules.

Therefore, the structure of cross-linked silicone formed is as follows:

Thus, option (B) is the correct answer.
Note:
It is important to note that the nature of the alkyl group present and the extent of cross linking is responsible to determine the nature of polymer formed. The nature of polymers may vary from oily liquids to solids having rubber like properties.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




