
Which of the following compound will give \[{{\text{S}}_{\text{N}}}1{\text{ and }}{{\text{S}}_{\text{N}}}2\] reactions with considerable rate?
I.\[{{\text{C}}_6}{{\text{H}}_5} - {\text{C}}{{\text{H}}_2} - {\text{Br}}\]
II.\[{\text{C}}{{\text{H}}_2} = {\text{CH}} - {\text{C}}{{\text{H}}_2} - {\text{Br}}\]
III.\[{\text{C}}{{\text{H}}_3} - {\text{CH}}\left( {{\text{Br}}} \right) - {\text{C}}{{\text{H}}_3}\]
IV.
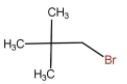
Select the correct answer according to the given codes below.
A.\[{\text{I, II and III}}\]
B.\[{\text{I, II and IV}}\]
C.\[{\text{II, III and IV}}\]
D.\[{\text{I, III and IV}}\]

Answer
573.9k+ views
Hint: The secondary alkyl halide reacts equally with the \[{{\text{S}}_{\text{N}}}1{\text{ and }}{{\text{S}}_{\text{N}}}2\] reaction. If the carbocation is stabilised by the resonance then ${{\text{S}}_{\text{N}}}1$ is more preferred.
Complete step by step answer:
The conversion of haloalkanes to alcohol is done by \[{{\text{S}}_{\text{N}}}1{\text{ and }}{{\text{S}}_{\text{N}}}2\] reactions using hydroxide ion as reagent in aqueous medium.
\[{{\text{S}}_{\text{N}}}1\] reaction is also known as nucleophilic substitution unimolecular reaction. It includes two steps. Tertiary carbocations are most stable and hence the tertiary alkyl halides are more reactive towards \[{{\text{S}}_{\text{N}}}1\] reactions followed by secondary and primary carbocation.
\[{{\text{S}}_{\text{N}}}2\] reaction is also known as nucleophilic substitution bimolecular reaction. It is a one step reaction and no intermediate forms in between. Hence, the rate of reaction depends upon the steric hindrance. Since primary alkyl halides are least sterically hindered and hence they are very much reactive towards the \[{{\text{S}}_{\text{N}}}2\] reaction followed by secondary and primary.
The above molecule is a typical tertiary alkyl halide and hence will always go for \[{{\text{S}}_{\text{N}}}1\] reaction because of the formation of stable carbocation.
\[{\text{C}}{{\text{H}}_3} - {\text{CH}}\left( {{\text{Br}}} \right) - {\text{C}}{{\text{H}}_3}\]
This is secondary alkyl halide and hence it will react equally with both the mechanism and have almost the same rate toward both.
\[{\text{C}}{{\text{H}}_2} = {\text{CH}} - {\text{C}}{{\text{H}}_2} - {\text{Br}}\]
\[{{\text{C}}_6}{{\text{H}}_5} - {\text{C}}{{\text{H}}_2} - {\text{Br}}\]
No doubt both of them are primary alkyl halide and should for \[{{\text{S}}_{\text{N}}}2\] reaction mechanism because of low steric hindrance, but the carbocation formed is stabilised by the resonance of phenol group and alkene. So they both will react at considerable rate with \[{{\text{S}}_{\text{N}}}1{\text{ and }}{{\text{S}}_{\text{N}}}2\] mechanism.
Hence, the correct option is A.
Note:
Rate of reaction in case of \[{{\text{S}}_{\text{N}}}1\] depends only on haloalkane, not on nucleophiles. Rate of \[{{\text{S}}_{\text{N}}}2\] reaction depends upon the, nature of the solvent, structure of the substrate, nature of the nucleophile and Effect of leaving-group.
Complete step by step answer:
The conversion of haloalkanes to alcohol is done by \[{{\text{S}}_{\text{N}}}1{\text{ and }}{{\text{S}}_{\text{N}}}2\] reactions using hydroxide ion as reagent in aqueous medium.
\[{{\text{S}}_{\text{N}}}1\] reaction is also known as nucleophilic substitution unimolecular reaction. It includes two steps. Tertiary carbocations are most stable and hence the tertiary alkyl halides are more reactive towards \[{{\text{S}}_{\text{N}}}1\] reactions followed by secondary and primary carbocation.
\[{{\text{S}}_{\text{N}}}2\] reaction is also known as nucleophilic substitution bimolecular reaction. It is a one step reaction and no intermediate forms in between. Hence, the rate of reaction depends upon the steric hindrance. Since primary alkyl halides are least sterically hindered and hence they are very much reactive towards the \[{{\text{S}}_{\text{N}}}2\] reaction followed by secondary and primary.

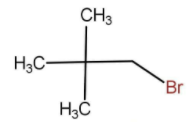
The above molecule is a typical tertiary alkyl halide and hence will always go for \[{{\text{S}}_{\text{N}}}1\] reaction because of the formation of stable carbocation.
\[{\text{C}}{{\text{H}}_3} - {\text{CH}}\left( {{\text{Br}}} \right) - {\text{C}}{{\text{H}}_3}\]
This is secondary alkyl halide and hence it will react equally with both the mechanism and have almost the same rate toward both.
\[{\text{C}}{{\text{H}}_2} = {\text{CH}} - {\text{C}}{{\text{H}}_2} - {\text{Br}}\]
\[{{\text{C}}_6}{{\text{H}}_5} - {\text{C}}{{\text{H}}_2} - {\text{Br}}\]
No doubt both of them are primary alkyl halide and should for \[{{\text{S}}_{\text{N}}}2\] reaction mechanism because of low steric hindrance, but the carbocation formed is stabilised by the resonance of phenol group and alkene. So they both will react at considerable rate with \[{{\text{S}}_{\text{N}}}1{\text{ and }}{{\text{S}}_{\text{N}}}2\] mechanism.
Hence, the correct option is A.
Note:
Rate of reaction in case of \[{{\text{S}}_{\text{N}}}1\] depends only on haloalkane, not on nucleophiles. Rate of \[{{\text{S}}_{\text{N}}}2\] reaction depends upon the, nature of the solvent, structure of the substrate, nature of the nucleophile and Effect of leaving-group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




