
Which of the following complexes is not chelate?
A. Bis(dimethylglyoximato)nickel(II)
B. Potassium ethylenediamine tetra thiocyanato chromate(III)
C. Tetraamminecarbonatocobalt(III) nitrate
D. Trans-glycinato platinum(II)
Answer
552.3k+ views
Hint: Chelate, any of a class of coordination or complex mixes consisting of a focal metal iota appended to an enormous atom, called a ligand, in a cyclic or ring structure. A case of a chelate ring happens in the ethylenediamine-cadmium complex: Chelate, Coordination compound.
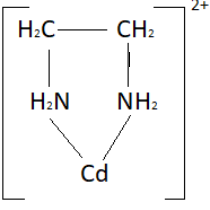
The ethylenediamine ligand has two purposes of connection to the cadmium particle, subsequently framing a ring; it is known as a di-dentate ligand. (Three ethylenediamine ligands can append to the \[C{d^{2 + }}\] particle, every one shaping a ring as portrayed above.) Ligands that can join to similar metal particles at least two focuses are known as polydentate ligands. All polydentate ligands are chelating specialists.
Complete step by step answer:
A chelate is a ring shaped structure, where the central atom is surrounded by every side and the structure formed is called chelate. In option (c), ammine (\[N{H_3}\]) and carbon monoxide (\[CO\]) both are monodentate ligands so they does not form a chelate.
Monodentate ligand is a ligand that has just a single particle that organizes legitimately to the focal iota in a complex. For instance, smelling salts and chloride particle are monodentate ligands of copper in the buildings \[{\left[ {Cu\left( {NH3} \right)6} \right]^{2 + }}\] and \[{\left[ {CuCl6} \right]^{2 + }}\].
Types of Ligands:
Unidentate ligands: Ligands with just a single giver molecule, for example \[N{H_3}\], \[Cl - ,{\text{ }}F - \] and so on Bidentate ligands: Ligands with two benefactor molecules, for example ethylenediamine, \[{C_2}{O_4}^{2 - }\] (oxalate particle) and so forth.
Tridentate ligands: Ligands which have three contributor molecules for each ligand, for example (dien) diethyl triamine.
Hexadentate ligands: Ligands which have six giver molecules for every ligand, for example EDTA.
Chelating Ligands: Multidentate ligand at the same time co-ordinating to a metal particle through more than one site is called chelating ligand. These ligands produce a ring like structure called chelate. Chelation builds the strength of complex. This impact is called chelation impact. Hence, option (c) is correct.
So, the correct answer is Option C.
Note: A ligand is a particle or atom that ties to a focal metal iota to frame a complex (on the other hand known as a coordination substance). Ligands are normally considered as electron benefactors pulled into the metal at the focal point of the complex.

The ethylenediamine ligand has two purposes of connection to the cadmium particle, subsequently framing a ring; it is known as a di-dentate ligand. (Three ethylenediamine ligands can append to the \[C{d^{2 + }}\] particle, every one shaping a ring as portrayed above.) Ligands that can join to similar metal particles at least two focuses are known as polydentate ligands. All polydentate ligands are chelating specialists.
Complete step by step answer:
A chelate is a ring shaped structure, where the central atom is surrounded by every side and the structure formed is called chelate. In option (c), ammine (\[N{H_3}\]) and carbon monoxide (\[CO\]) both are monodentate ligands so they does not form a chelate.
Monodentate ligand is a ligand that has just a single particle that organizes legitimately to the focal iota in a complex. For instance, smelling salts and chloride particle are monodentate ligands of copper in the buildings \[{\left[ {Cu\left( {NH3} \right)6} \right]^{2 + }}\] and \[{\left[ {CuCl6} \right]^{2 + }}\].
Types of Ligands:
Unidentate ligands: Ligands with just a single giver molecule, for example \[N{H_3}\], \[Cl - ,{\text{ }}F - \] and so on Bidentate ligands: Ligands with two benefactor molecules, for example ethylenediamine, \[{C_2}{O_4}^{2 - }\] (oxalate particle) and so forth.
Tridentate ligands: Ligands which have three contributor molecules for each ligand, for example (dien) diethyl triamine.
Hexadentate ligands: Ligands which have six giver molecules for every ligand, for example EDTA.
Chelating Ligands: Multidentate ligand at the same time co-ordinating to a metal particle through more than one site is called chelating ligand. These ligands produce a ring like structure called chelate. Chelation builds the strength of complex. This impact is called chelation impact. Hence, option (c) is correct.
So, the correct answer is Option C.
Note: A ligand is a particle or atom that ties to a focal metal iota to frame a complex (on the other hand known as a coordination substance). Ligands are normally considered as electron benefactors pulled into the metal at the focal point of the complex.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




