
Which of the following alcohol will have the fastest rate of dehydration?
A)
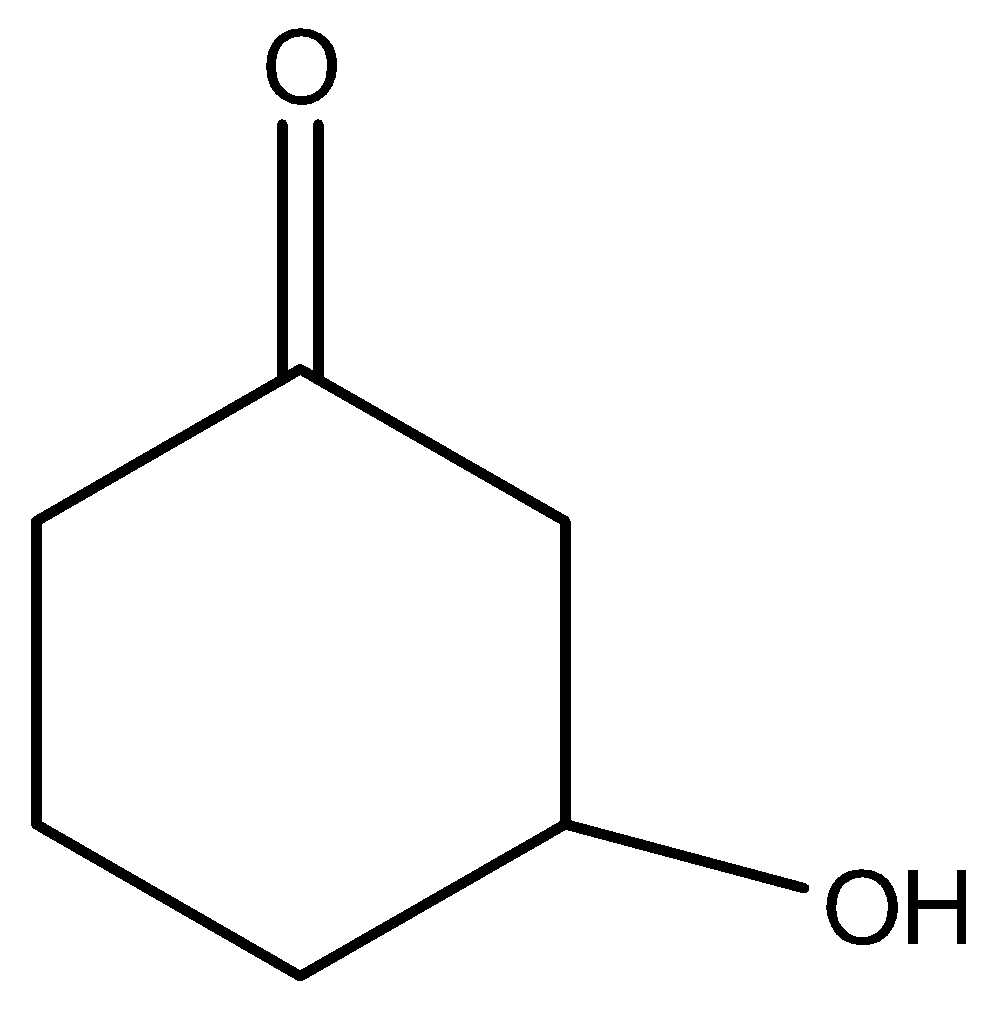
B)
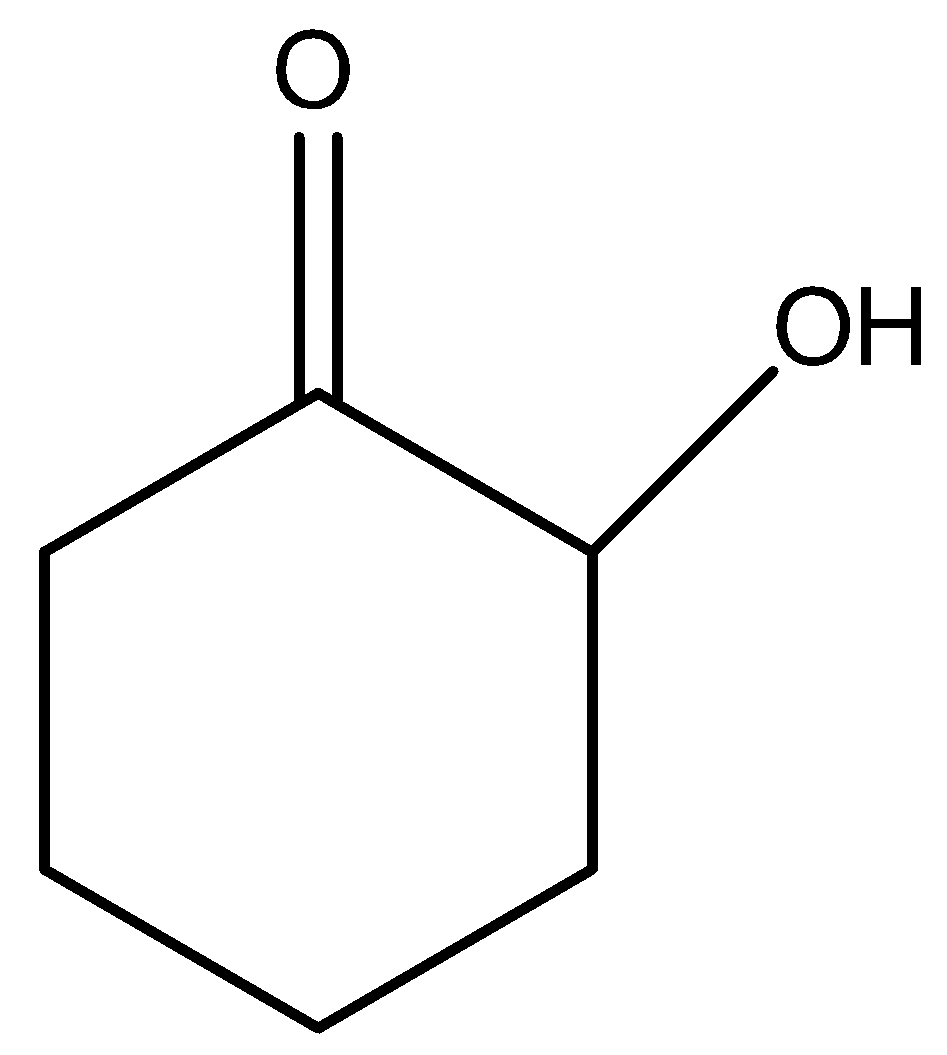
C)

D)





Answer
565.5k+ views
Hint: You should first know what dehydration is. When alcohol reacts with protic acids or dehydrating agents, it tends to lose a molecule of water in order to form alkenes. This reaction is known as dehydration reaction. To remove a water molecule, we need to remove an OH and H atom. When the carbocation intermediate formed in a reaction is stable then, the reaction is faster.
Complete answer:
Alcohols upon reaction with protic acids, like conc. ${H_2}S{O_4}$, tends to lose a water molecule and forms alkenes as products. These reactions are termed as dehydration of alcohols.
Let us do dehydration reaction of each alcohol given in options one by one:
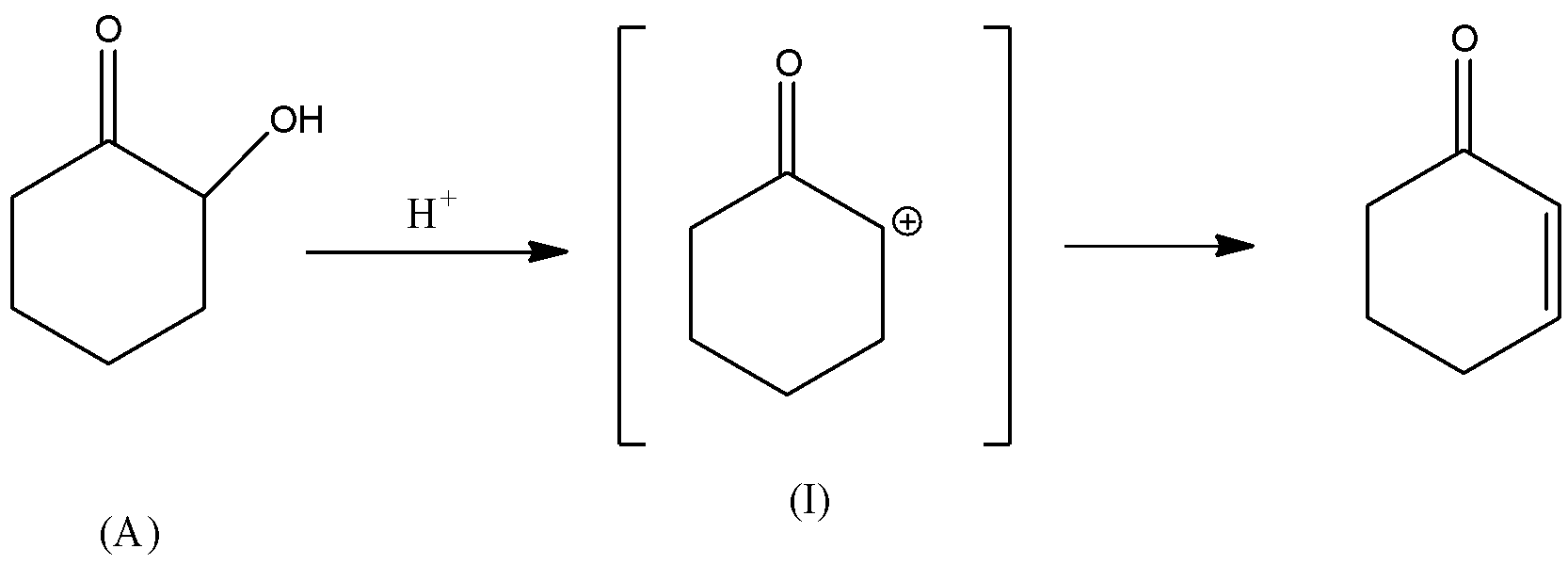

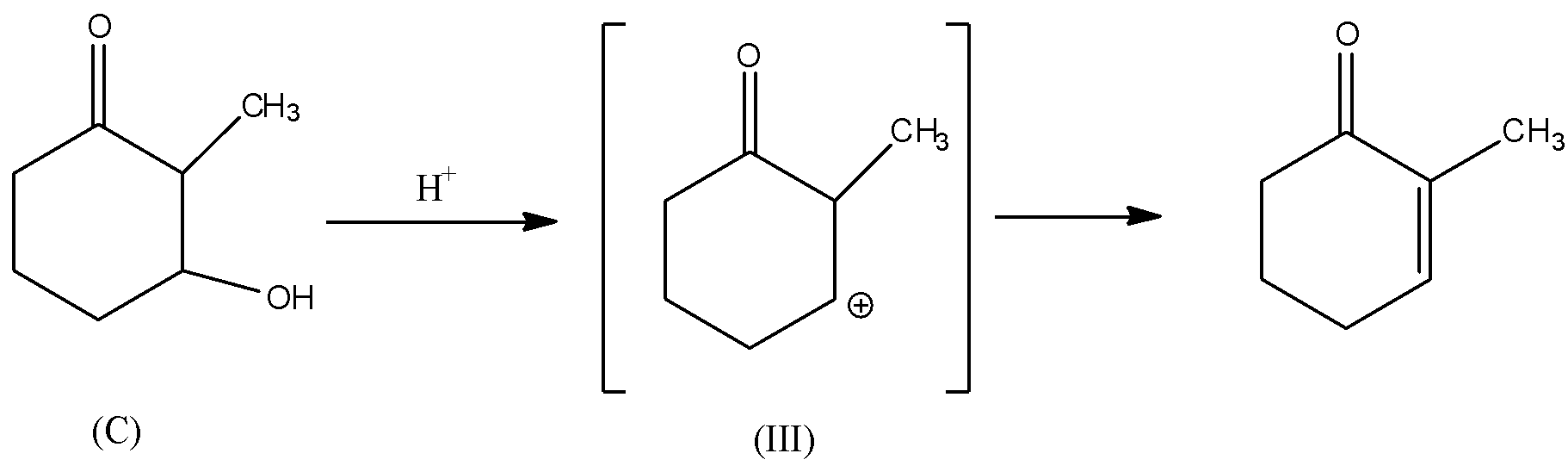
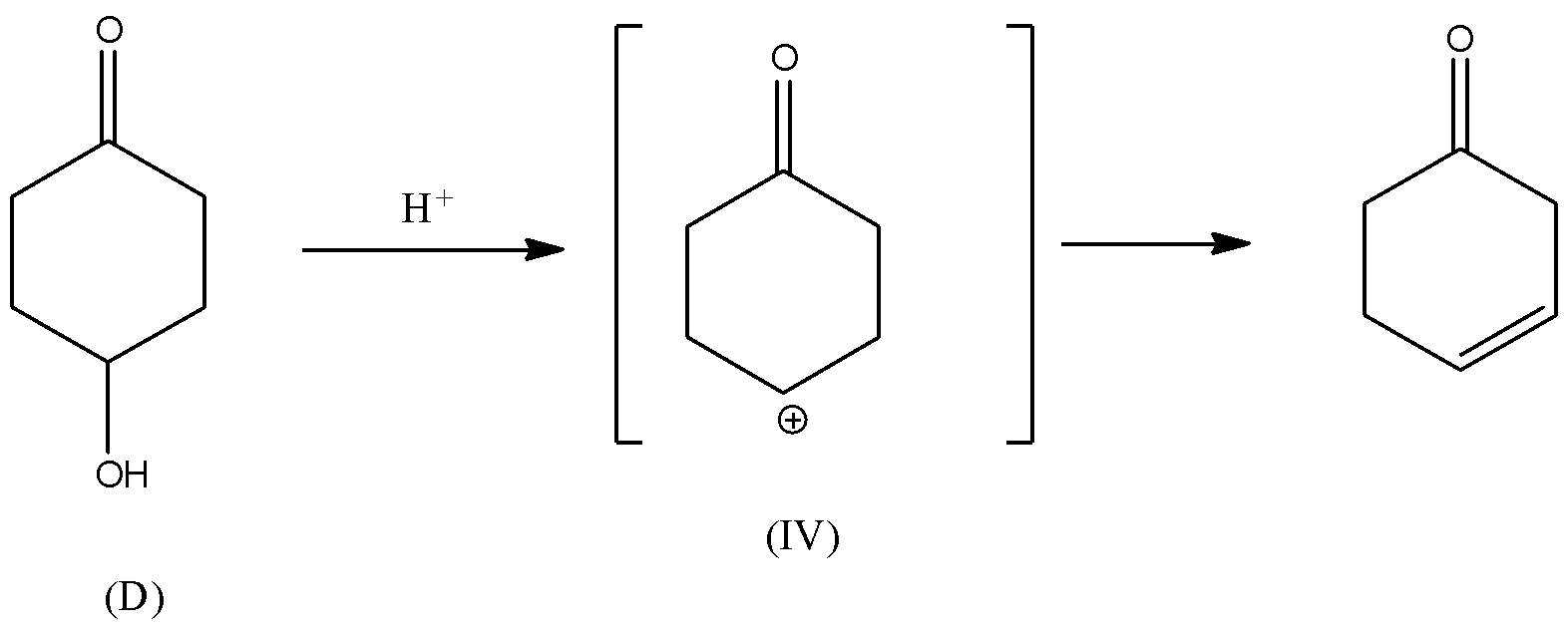
Rate of dehydration reaction depends upon the stability of the carbocation (${C^ \oplus }$) intermediate formed during the reaction. If the carbocation formed is stable, then the dehydration reaction of alcohol is faster because the intermediate will form at the faster rate. There will be rearrangement of the hydrogen atom in the intermediate (III). Also, carbocation intermediate (III) will be in conjugation with the double bond of carbonyl group. Hence, carbocation (III) will be the most stable due to conjugation with double bonds. Thus, it is thermodynamically favoured and hence, the rate of formation of carbocation will be faster. Consequently, the rate of dehydration of alcohol (C) will be faster.
In all other intermediates i.e., (I), (II), and (IV), the carbocation is not in resonance or any conjugation with the double bond. Hence, these carbocations are relatively less stable than the carbocation (III). Thus, the dehydration reaction of alcohols (A), (B) and (D) will be relatively slower than the dehydration reaction of (C).
Hence, alcohol given in option C will have the fastest rate of dehydration.
Thus, option C is the answer.
Note:
Dehydration reaction is an example of an elimination reaction. Its rate varies for primary $({1^o})$, secondary $({2^o})$ and tertiary $({3^o})$ alcohols. This variation of rate depends on the stability of carbocation generated. Since the carbocation is most stable in the case of tertiary alcohols, the rate of dehydration of alcohols is highest for tertiary alcohols in comparison to secondary and primary alcohols.
Complete answer:
Alcohols upon reaction with protic acids, like conc. ${H_2}S{O_4}$, tends to lose a water molecule and forms alkenes as products. These reactions are termed as dehydration of alcohols.
Let us do dehydration reaction of each alcohol given in options one by one:




Rate of dehydration reaction depends upon the stability of the carbocation (${C^ \oplus }$) intermediate formed during the reaction. If the carbocation formed is stable, then the dehydration reaction of alcohol is faster because the intermediate will form at the faster rate. There will be rearrangement of the hydrogen atom in the intermediate (III). Also, carbocation intermediate (III) will be in conjugation with the double bond of carbonyl group. Hence, carbocation (III) will be the most stable due to conjugation with double bonds. Thus, it is thermodynamically favoured and hence, the rate of formation of carbocation will be faster. Consequently, the rate of dehydration of alcohol (C) will be faster.
In all other intermediates i.e., (I), (II), and (IV), the carbocation is not in resonance or any conjugation with the double bond. Hence, these carbocations are relatively less stable than the carbocation (III). Thus, the dehydration reaction of alcohols (A), (B) and (D) will be relatively slower than the dehydration reaction of (C).
Hence, alcohol given in option C will have the fastest rate of dehydration.
Thus, option C is the answer.
Note:
Dehydration reaction is an example of an elimination reaction. Its rate varies for primary $({1^o})$, secondary $({2^o})$ and tertiary $({3^o})$ alcohols. This variation of rate depends on the stability of carbocation generated. Since the carbocation is most stable in the case of tertiary alcohols, the rate of dehydration of alcohols is highest for tertiary alcohols in comparison to secondary and primary alcohols.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




