
Which of the following acid does not have$ - COOH$group?
(A) Picric acid
(B) Ethanoic acid
(C) Benzoic acid
(D) Salicylic acid
Answer
592.2k+ views
Hint: Carboxylic compound contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is $R - COOH$ where R is alkyl group.
Complete step by step answer:
Let us draw structures of given compound
(A) Picric Acid:
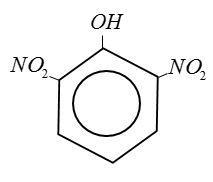
(B) Benzoic Acid:
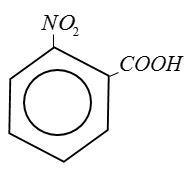
(C) Ethanoic Acid: $C{H_3}COOH$
(D) Salicylic Acid:
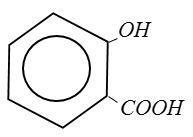
From above structure it is clear that Picric Acid no$ - COOH$group.
IUPAC name of picric acid 2, 4, 6 trinitro phenol. [TNP].
Picric word comes from Greek word; it means ‘bitter’.
Due to its bitter taste it is one of the most acidic phenols.
Other names of picric acid are earbazotic acid phenol triturate, picro nitric acid, luddite etc.
Acidic nature of picric acid:
Due to the resonant structure of picric acid it is very stable. Conjugate base does not combine with lost ${H^ + }.$ This is why TNP is a strong acid even though it does not contain$ - COOH$group.
The $pKa$ value of picric acid is $0.38.$
Also picric acid contains three Electrons Withdrawing group $ - N{O_2}$ on 2, 4, 6 position.
Electron withdrawing groups increase acidic character.
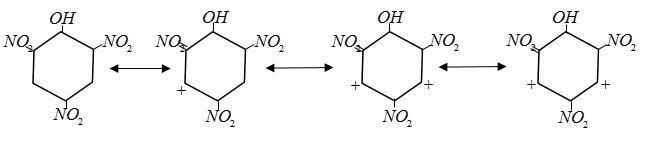
Therefore, from the above explanation the correct option is (A) Picric acid.
Additional Information:
Picric acid is yellow, odorless crystalline solid. It is prepared by reaction of phenol with concentration $HN{O_3}$ acid.
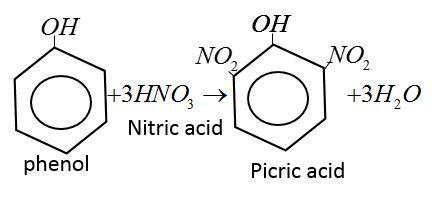
Note:
2, 4, 6 trinitrophenol is called picric acid because it is a strong acid containing three strong electron withdrawing group $ - N{O_2}.$like organic acid it easily gives $[{H^ + }].$
Complete step by step answer:
Let us draw structures of given compound
(A) Picric Acid:

(B) Benzoic Acid:

(C) Ethanoic Acid: $C{H_3}COOH$
(D) Salicylic Acid:

From above structure it is clear that Picric Acid no$ - COOH$group.
IUPAC name of picric acid 2, 4, 6 trinitro phenol. [TNP].
Picric word comes from Greek word; it means ‘bitter’.
Due to its bitter taste it is one of the most acidic phenols.
Other names of picric acid are earbazotic acid phenol triturate, picro nitric acid, luddite etc.
Acidic nature of picric acid:
Due to the resonant structure of picric acid it is very stable. Conjugate base does not combine with lost ${H^ + }.$ This is why TNP is a strong acid even though it does not contain$ - COOH$group.
The $pKa$ value of picric acid is $0.38.$
Also picric acid contains three Electrons Withdrawing group $ - N{O_2}$ on 2, 4, 6 position.
Electron withdrawing groups increase acidic character.

Therefore, from the above explanation the correct option is (A) Picric acid.
Additional Information:
Picric acid is yellow, odorless crystalline solid. It is prepared by reaction of phenol with concentration $HN{O_3}$ acid.

Note:
2, 4, 6 trinitrophenol is called picric acid because it is a strong acid containing three strong electron withdrawing group $ - N{O_2}.$like organic acid it easily gives $[{H^ + }].$
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




