
Which is more stable and why?

Answer
573.3k+ views
Hint: Cyclohexane is a saturated compound while benzene is an unsaturated compound. Cyclohexane exists mostly in chair form and benzene is an aromatic compound.
Complete answer:
Cyclohexane is more stable compared to benzene. Although everyone would be thinking its benzene which is more stable but it’s not the case. The reason for thinking benzene to be more stable is due to the following reason:
- benzene is less reactive
- It is an aromatic compound.
- it obeys Huckel rule
- It has many resonance structures.
But it’s not the case. If we learn about Heat of formation / combustion graph we would get a clear idea. What is standard heat of formation? Standard Heat of formation is also known as standard enthalpy of formation. Standard enthalpy of formation is the change in enthalpy during formation of one mole of the substance. Lower the heat of formation, more stable is the compound.
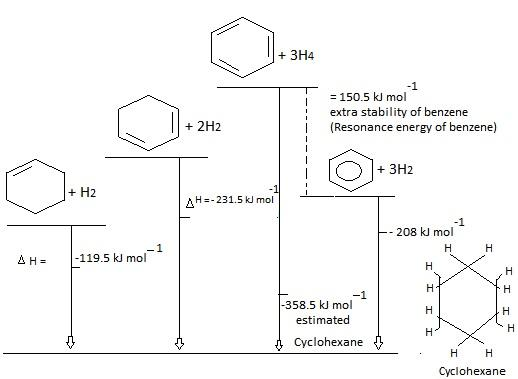
From the above graph, it's very clear that cyclohexane is more stable compared to benzene. Because cyclohexane has 50 kcals/mol less energy compared to benzene. As we know lower the heat of formation more stable the compound.
Additional information:
Comparison of benzene and cyclohexane.
Note: - Due to benzene’s aromaticity, resonance structure and less reactive nature it was thought to be very stable that cyclohexane.
- The stability of the compounds is given in the increasing order:
Cyclohexane>Cyclohexene>Benzene>1,3-Cyclohexadiene
Complete answer:
Cyclohexane is more stable compared to benzene. Although everyone would be thinking its benzene which is more stable but it’s not the case. The reason for thinking benzene to be more stable is due to the following reason:
- benzene is less reactive
- It is an aromatic compound.
- it obeys Huckel rule
- It has many resonance structures.
But it’s not the case. If we learn about Heat of formation / combustion graph we would get a clear idea. What is standard heat of formation? Standard Heat of formation is also known as standard enthalpy of formation. Standard enthalpy of formation is the change in enthalpy during formation of one mole of the substance. Lower the heat of formation, more stable is the compound.

From the above graph, it's very clear that cyclohexane is more stable compared to benzene. Because cyclohexane has 50 kcals/mol less energy compared to benzene. As we know lower the heat of formation more stable the compound.
Additional information:
Comparison of benzene and cyclohexane.
| BENZENE | CYCLOHEXANE |
| Molecular formula- \[{{C}_{6}}{{H}_{6}}\] | Molecular formula- \[{{C}_{6}}{{H}_{12}}\] |
| Hybridization- \[s{{p}^{2}}\] | Hybridization- \[s{{p}^{3}}\] |
| Aromatic structure | Not aromatic, but cyclic structure |
| Presence of α and β bonds | Presence of only α bonds. |
Note: - Due to benzene’s aromaticity, resonance structure and less reactive nature it was thought to be very stable that cyclohexane.
- The stability of the compounds is given in the increasing order:
Cyclohexane>Cyclohexene>Benzene>1,3-Cyclohexadiene
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




