
Which form(s) of cyclohexane is/are free from angle strain?
(A) Chair form
(B) Boat form
(C) Twist boat form
(D) All of the above
Answer
593.1k+ views
Hint: It’s a form where the molecule has a plane made out of four carbon atoms. The other two carbon atoms are either below or above this plane.
Complete answer:
We define angle strain as a phenomenon which arises when the angle between bonds are either compressed or expanded from their optimal value. Say for example, you have a carbon atom which is $s{{p}^{3}}$ hybridised. You already know that its geometry would be tetrahedral and the optimal angle between the bonds is $109.5{}^\circ $. But sometimes this is not possible due to intervention of some other chemical group or due to non-bonding electrons. Now this puts a strain on the bonds because the angles are not positioned as they should be; the strain can therefore be defined as the excess energy these bonds possess, which is waiting to be released at any opportunity. In the cycloalkanes particularly, there is a permanent angle strain on ring structures which have less than six carbon atoms.
The cyclohexane, which we have here, is a structure that has many possible conformations. A conformer is an isomer which results due to the change in structure around a single bond. A cyclohexane can conform between seven structures. They are arranged serially below:
\[\text{Chair}\to \text{Half-chair}\to \text{Twisted boat}\to \text{Boat}\to \text{Twisted boat}\to \text{Half-chair}\to \text{Chair}\]
The most stable conformer among the above mentioned conformers is the chair form. The first and the last chair form differ in their spatial arrangement of atoms. They are always in dynamic equilibrium with each other. It is shown below:
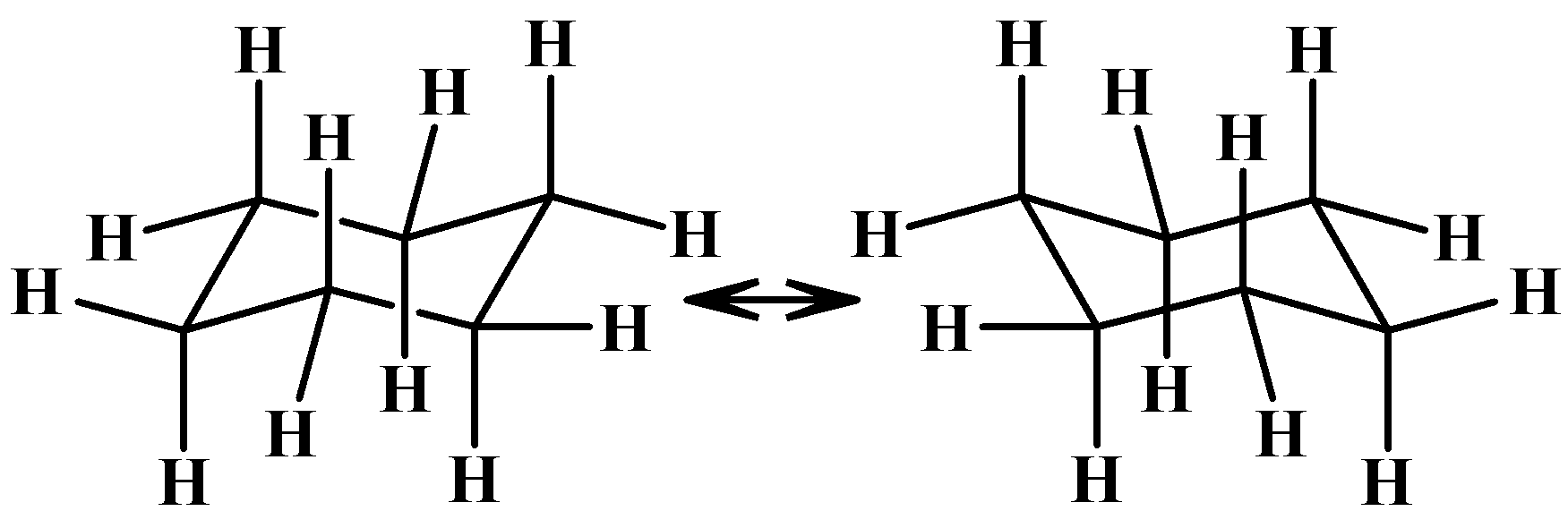
There is the least angle strain in this form because the bond angles are nearly equal to that of the optimal value of a$s{{p}^{3}}$hybridised carbon atom, unlike the other forms.
Hence our answer to the above question is option (A) Chair form.
Note: There are three types of strains that scientists try to define for every chemical compound they study. They are torsional strain, angle strain and steric strain. The chair conformation of cyclohexane has the least value in all kinds of strain, making it the optimum form of the compound. That means, in a room full of cyclohexane, 99.99% of molecules would be in this conformation.
Complete answer:
We define angle strain as a phenomenon which arises when the angle between bonds are either compressed or expanded from their optimal value. Say for example, you have a carbon atom which is $s{{p}^{3}}$ hybridised. You already know that its geometry would be tetrahedral and the optimal angle between the bonds is $109.5{}^\circ $. But sometimes this is not possible due to intervention of some other chemical group or due to non-bonding electrons. Now this puts a strain on the bonds because the angles are not positioned as they should be; the strain can therefore be defined as the excess energy these bonds possess, which is waiting to be released at any opportunity. In the cycloalkanes particularly, there is a permanent angle strain on ring structures which have less than six carbon atoms.
The cyclohexane, which we have here, is a structure that has many possible conformations. A conformer is an isomer which results due to the change in structure around a single bond. A cyclohexane can conform between seven structures. They are arranged serially below:
\[\text{Chair}\to \text{Half-chair}\to \text{Twisted boat}\to \text{Boat}\to \text{Twisted boat}\to \text{Half-chair}\to \text{Chair}\]
The most stable conformer among the above mentioned conformers is the chair form. The first and the last chair form differ in their spatial arrangement of atoms. They are always in dynamic equilibrium with each other. It is shown below:

There is the least angle strain in this form because the bond angles are nearly equal to that of the optimal value of a$s{{p}^{3}}$hybridised carbon atom, unlike the other forms.
Hence our answer to the above question is option (A) Chair form.
Note: There are three types of strains that scientists try to define for every chemical compound they study. They are torsional strain, angle strain and steric strain. The chair conformation of cyclohexane has the least value in all kinds of strain, making it the optimum form of the compound. That means, in a room full of cyclohexane, 99.99% of molecules would be in this conformation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




