
Which amino acids have an aromatic ring?
A. Alanine
B. Glycine
C. Tyrosine
D. Lysine
Answer
233.1k+ views
Hint: An aromatic substance is referred to as having an aromatic ring. The molecules that make up proteins have both an acid and an ammoniated group. A peptide bond is created when the acid and ammoniated group lose water. The amino acids are found in zwitter ionic form, where each molecule has a distinct charge.
Complete Step by Step Solution:
An aromatic ring makes up aromatic amino acids. Among the 20 present, there are 3 aromatic amino acids: phenylaniline, tyrosine, and tryptophan. All of the amino acids are necessary. We must obtain essential amino acids through dietary supplements since our bodies cannot generate them. Different illnesses are brought on by a deficiency of necessary amino acids. The absence of phenylaniline hydrolysis, which results in phenylketonuria, is typically the cause of phenylaniline insufficiency. Stunted growth results from tyrosine insufficiency, and Blue diaper syndrome from tryptophan shortage. Tyrosine has a 4-hydroxyphenylalanine structure, therefore an aniline group has a phenyl ring that is aromatic by nature. Foods high in protein are where tyrosine is found. The structure of Tyrosine is given as:
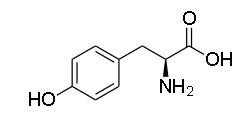
Hence, the correct option is C. Tyrosine.
Additional Information: The structure of the other three compounds are as follows:
The structure of Lysine:
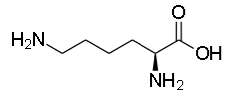
The structure of Glycine:
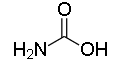
The structure of Alanine:
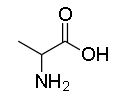
Note: The thing to note here is that there are only three aromatic amino acids among the 20 amino acids. The remaining three are Glycine, Alanine and Lysine and they all form a straight chain and no aromatic rings are there in their structure.
Complete Step by Step Solution:
An aromatic ring makes up aromatic amino acids. Among the 20 present, there are 3 aromatic amino acids: phenylaniline, tyrosine, and tryptophan. All of the amino acids are necessary. We must obtain essential amino acids through dietary supplements since our bodies cannot generate them. Different illnesses are brought on by a deficiency of necessary amino acids. The absence of phenylaniline hydrolysis, which results in phenylketonuria, is typically the cause of phenylaniline insufficiency. Stunted growth results from tyrosine insufficiency, and Blue diaper syndrome from tryptophan shortage. Tyrosine has a 4-hydroxyphenylalanine structure, therefore an aniline group has a phenyl ring that is aromatic by nature. Foods high in protein are where tyrosine is found. The structure of Tyrosine is given as:

Hence, the correct option is C. Tyrosine.
Additional Information: The structure of the other three compounds are as follows:
The structure of Lysine:

The structure of Glycine:

The structure of Alanine:

Note: The thing to note here is that there are only three aromatic amino acids among the 20 amino acids. The remaining three are Glycine, Alanine and Lysine and they all form a straight chain and no aromatic rings are there in their structure.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




