
What type of stoichiometric defect is shown by AgCl?
Answer
602.4k+ views
Hint: AgCl is a chemical compound known as silver chloride. It is very less soluble in water. Each silver ion in the lattice’s interior binds with six chloride ions, and each chloride ion in the interior binds with six silver ions. Those ions on the lattice’s surface or edges bind to fewer than six ions and carry a partial charge.
Complete step by step answer:
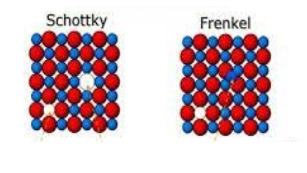
-The two types of defects in any lattice structure are: Schottky defect and Frenkel defect. Schottky defect arises when equal numbers of cations and anions are missing from the lattice. It is a common defect in ionic compounds with high coordination numbers where both cations and anions are of the same size. Due to this, the density of the crystal decreases and it starts to conduct electricity to a smaller extent.
-On the other hand, Frenkel defect arises when some of the ions of the lattice occupy interstitial sites leaving the previous spaces vacant. This defect is found in ionic compounds in which the anion is much larger than the cation in size. The density of the compound does not change due to this defect and the overall composition remains the same.
-In AgCl, as the anion is much larger than the cation, it shows Frenkel defect. The silver ions are much smaller than the chloride ions. So, the silver ions occupy the interstitial sites, leaving a corresponding number of normal lattice sites vacant.
Hence, the correct answer is Frenkel defect.
Note:
Remember that the Frenkel defect does not change the composition of the compound as no ions leave the lattice in this defect. However, in Schottky defect, equal numbers of cations and anions leave the lattice.
Complete step by step answer:
-The two types of defects in any lattice structure are: Schottky defect and Frenkel defect. Schottky defect arises when equal numbers of cations and anions are missing from the lattice. It is a common defect in ionic compounds with high coordination numbers where both cations and anions are of the same size. Due to this, the density of the crystal decreases and it starts to conduct electricity to a smaller extent.
-On the other hand, Frenkel defect arises when some of the ions of the lattice occupy interstitial sites leaving the previous spaces vacant. This defect is found in ionic compounds in which the anion is much larger than the cation in size. The density of the compound does not change due to this defect and the overall composition remains the same.
-In AgCl, as the anion is much larger than the cation, it shows Frenkel defect. The silver ions are much smaller than the chloride ions. So, the silver ions occupy the interstitial sites, leaving a corresponding number of normal lattice sites vacant.

Hence, the correct answer is Frenkel defect.
Note:
Remember that the Frenkel defect does not change the composition of the compound as no ions leave the lattice in this defect. However, in Schottky defect, equal numbers of cations and anions leave the lattice.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Coming together federation is practiced in A India class 12 social science CBSE

How was the Civil Disobedience Movement different from class 12 social science CBSE




