
The total number of isomers, considering both the structural and stereoisomers, of a cyclic ether with the molecular formula ${{C}_{4}}{{H}_{8}}O$ is:
Answer
578.4k+ views
Hint: Think about all the structural isomers that can be formed by varying the number of atoms that will be a part of the ring and then check if they have a chiral center or not to find the stereoisomers.
Complete step by step solution:
To solve this question, we will first look at all the structural isomers by varying the number of atoms that are included in the ring and rearranging the remaining atoms as substituents. We will then check if the molecules have a plane of symmetry and a chiral carbon to form stereoisomers. We can have cyclic ethers with 2, 3, or 4 carbon atoms. The remaining carbon atoms will be arranged as branches to the ring.
- For a 3 membered ring
In a 3-membered ring, we have 2 left over carbons. We can arrange them directly as an ethyl group or as 2 methyl groups. If an ethyl group is considered, only 1 isomer is possible. If we consider 2 methyl groups, then we can form 2 isomers. The structures will be as follows:
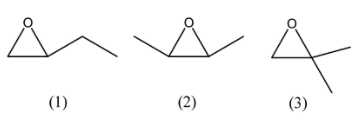
Here, we can see that the first structure has a chiral centre and does not have a plane of symmetry, hence it will have two stereoisomers. In the third structure, we have a chiral centre but we also have a plane of symmetry, so it will not have any stereoisomers, but if we change the positioning of one of the methyl groups, then the images will not have a plane of symmetry and we can have 2 stereoisomers. So, the fourth structural isomer that can be formed with a 3-membered ring is:
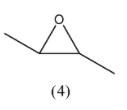
We can see here that this molecule does not have a plane of symmetry so we can have 2 stereoisomers. Among all the structural isomers of a 3 membered ring of the cyclic ether, structures (1) and (4) will have structural isomers. They are as follows:
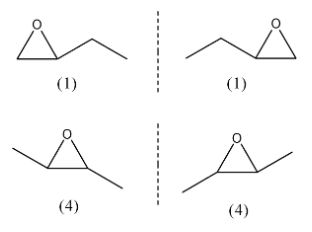
So, we have a total of 6 isomers of the cyclic ether with the formula ${{C}_{4}}{{H}_{8}}O$ with a 3-membered ring.
- For a 4-membered ring
With a 4-membered we only have only a methyl group that we can use to attach as a substituent to the members of the ring. Out of those, we cannot attach it to the oxygen molecule but the other carbon atoms. One of the isomers will have the methyl group attached to a carbon atom that is adjacent to the oxygen atom and the other isomer will have the methyl group attached to the carbon atom that is placed opposite to the oxygen atom in the ring. The structures are as follows:
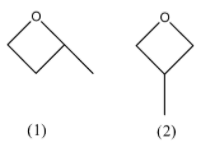
In the first structure, we have a chiral point and do not have a plane of symmetry, so the first structure will have another stereoisomer. In the second structure, we have a plane of symmetry so it will not have stereoisomers. The stereoisomer of the first structure will be:
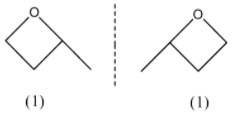
Now, we have 3 isomers with a 4 membered ring.
- For a 5-membered ring
If we consider a 5-membered ring, we will not have any substituents that we can attach to the members of the ring and we will naturally only have one isomer. The structure of this molecule will be:
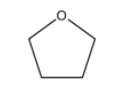
There are no other isomers possible for this structure.
Hence, we have a total of 10 structural and stereoisomers for a cyclic ether that has the molecular formula ${{C}_{4}}{{H}_{8}}O$.
Note: You can also check the stereoisomerism using the R and S configurations and nomenclature instead of trying to work it out manually. For example, in that case, the structure (2) in the 3-membered ring structures will have 3 stereoisomers with configurations (R,R), (R,S), and (S,S). Both the isomers of structure (4) will be included in this.
Complete step by step solution:
To solve this question, we will first look at all the structural isomers by varying the number of atoms that are included in the ring and rearranging the remaining atoms as substituents. We will then check if the molecules have a plane of symmetry and a chiral carbon to form stereoisomers. We can have cyclic ethers with 2, 3, or 4 carbon atoms. The remaining carbon atoms will be arranged as branches to the ring.
- For a 3 membered ring
In a 3-membered ring, we have 2 left over carbons. We can arrange them directly as an ethyl group or as 2 methyl groups. If an ethyl group is considered, only 1 isomer is possible. If we consider 2 methyl groups, then we can form 2 isomers. The structures will be as follows:

Here, we can see that the first structure has a chiral centre and does not have a plane of symmetry, hence it will have two stereoisomers. In the third structure, we have a chiral centre but we also have a plane of symmetry, so it will not have any stereoisomers, but if we change the positioning of one of the methyl groups, then the images will not have a plane of symmetry and we can have 2 stereoisomers. So, the fourth structural isomer that can be formed with a 3-membered ring is:

We can see here that this molecule does not have a plane of symmetry so we can have 2 stereoisomers. Among all the structural isomers of a 3 membered ring of the cyclic ether, structures (1) and (4) will have structural isomers. They are as follows:

So, we have a total of 6 isomers of the cyclic ether with the formula ${{C}_{4}}{{H}_{8}}O$ with a 3-membered ring.
- For a 4-membered ring
With a 4-membered we only have only a methyl group that we can use to attach as a substituent to the members of the ring. Out of those, we cannot attach it to the oxygen molecule but the other carbon atoms. One of the isomers will have the methyl group attached to a carbon atom that is adjacent to the oxygen atom and the other isomer will have the methyl group attached to the carbon atom that is placed opposite to the oxygen atom in the ring. The structures are as follows:

In the first structure, we have a chiral point and do not have a plane of symmetry, so the first structure will have another stereoisomer. In the second structure, we have a plane of symmetry so it will not have stereoisomers. The stereoisomer of the first structure will be:

Now, we have 3 isomers with a 4 membered ring.
- For a 5-membered ring
If we consider a 5-membered ring, we will not have any substituents that we can attach to the members of the ring and we will naturally only have one isomer. The structure of this molecule will be:

There are no other isomers possible for this structure.
Hence, we have a total of 10 structural and stereoisomers for a cyclic ether that has the molecular formula ${{C}_{4}}{{H}_{8}}O$.
Note: You can also check the stereoisomerism using the R and S configurations and nomenclature instead of trying to work it out manually. For example, in that case, the structure (2) in the 3-membered ring structures will have 3 stereoisomers with configurations (R,R), (R,S), and (S,S). Both the isomers of structure (4) will be included in this.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




