
What is the number of valence electrons in the $C{{l}^{-}}$ion?
a.) 16
b.) 8
c.) 17
d.) 18
Answer
597.9k+ views
Hint: The number of valence electrons is the number of electrons present in the valence/last shell of the atom of the chemical substance. Also bear in mind that a charged anion’s atom has more electrons when compared to an atom of the ground state of the same element.
Complete step by step solution:
Let us first learn in detail the meaning of the terms valence shell and valence electrons before attempting to answer this question.
The Valence shell is the outermost shell of every element and the atoms of every element have different electronic configurations based on their atomic numbers which in turn refers to the distribution of electrons in various shells/orbits/energy levels of every atom.
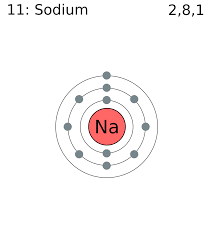
For example, Sodium with Atomic no.= 11 has electronic configuration K,L,M.
Now we know that the valence electrons of an atom are those electrons that are present in the outermost shell of an atom.
Valence electrons are crucial in Chemistry as they provide important insight into an element’s chemical properties: whether it is electronegative or electropositive in nature, and they also help indicate the bond order of a chemical compound i.e. the number of bonds that can be formed between two atoms.
Now let us apply these concepts to the $C{{l}^{-}}$ion:
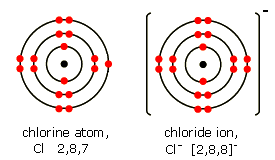 Analysing this figure and based on the definitions of valence shell and electrons, we observe that this anion has 8 valence electrons.
Analysing this figure and based on the definitions of valence shell and electrons, we observe that this anion has 8 valence electrons.
Therefore, the answer to this question is b) 8.
Note: Be very wary of the differences between Cl atoms in its ground state and the $C{{l}^{-}}$ ion. Also, be extremely careful about which shell of an atom in particular we refer to when speaking about the valence shell.
Complete step by step solution:
Let us first learn in detail the meaning of the terms valence shell and valence electrons before attempting to answer this question.
The Valence shell is the outermost shell of every element and the atoms of every element have different electronic configurations based on their atomic numbers which in turn refers to the distribution of electrons in various shells/orbits/energy levels of every atom.
For example, Sodium with Atomic no.= 11 has electronic configuration K,L,M.
Now we know that the valence electrons of an atom are those electrons that are present in the outermost shell of an atom.
Valence electrons are crucial in Chemistry as they provide important insight into an element’s chemical properties: whether it is electronegative or electropositive in nature, and they also help indicate the bond order of a chemical compound i.e. the number of bonds that can be formed between two atoms.

Now let us apply these concepts to the $C{{l}^{-}}$ion:

Therefore, the answer to this question is b) 8.
Note: Be very wary of the differences between Cl atoms in its ground state and the $C{{l}^{-}}$ ion. Also, be extremely careful about which shell of an atom in particular we refer to when speaking about the valence shell.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




