
The structure of $S{{O}_{3}}$ molecule in the gaseous phase contains:
(A) Only $\sigma $- bond between sulphur and oxygen
(B) $\sigma $ bonds and a (p$\pi $-p$\pi $) bonds between sulphur and oxygen
(C) $\sigma $bonds and a d$\pi $- p$\pi $) bonds between sulphur and oxygen
(D) $\sigma $ bonds and a (p$\pi $-p$\pi $) bonds and a ( d$\pi $- p$\pi $) bonds between sulphur and oxygen
Answer
573.6k+ views
Hint: To answer this question we should be aware when $\sigma $bonds, (p$\pi $-p$\pi $) bonds and ( d$\pi $- p$\pi $) bonds are formed. Valence electrons are the electrons that are present in the outermost shell of an atom upon this the covalency of the atom depends.
Complete answer:
Sulphur has six electrons in its outermost shell that is its covalency is six. Covalency of an atom is the number bonds an atom can make. In solid state the $S{{O}_{3}}$molecule exists in a cluster that is the number of$S{{O}_{3}}$ molecules bonded together to form a solid structure. In the gas phase the $S{{O}_{3}}$ molecule exists as a single molecule which is also said to exist in the free State.
$\sigma $- bond is formed by overlapping of atomic orbitals or hybrid orbitals in their axis.
For example: overlap of s-s, s-p,$s{{p}^{2}}$-$s{{p}^{2}}$ overlap along their axis.
\[\pi \]- bond is formed by the side to side overlap of molecular orbital or atomic orbital along a plane perpendicular to the plane. If the donating orbital is p and the orbital to which the electron pair is donated is p then it is known as P\[\pi \]-p\[\pi \].
Similarly, if the donating orbital is d and the orbital to which the electron pair is donated is p then it is known as d$\pi $- p$\pi $
Firstly, let's draw the Lewis structure of$S{{O}_{3}}$:
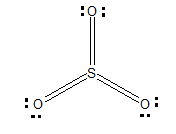
There are three delocalised \[\pi \] bonds, out of which one of them is P\[\pi \]-p\[\pi \]and other two are d$\pi $- p$\pi $. The sulphur is $d\pi -p\pi $hybridized.
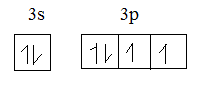
The electronic configuration of sulphur is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{4}}$.
Sulphur in ground state:
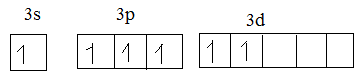
In excited state:
Oxygen has 6 electrons. So, the valence electrons of oxygen make bond with unpaired electrons.
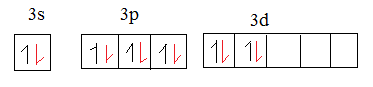
The 6 valence electrons in oxygen are of 2p orbital.
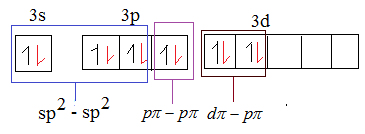
The red coloured electrons in the above mentioned diagram are the electrons of oxygen. The $s{{p}^{2}}$-$s{{p}^{2}}$ overlap forms $\sigma $ bond as sulphur in $S{{O}_{3}}$ is $s{{p}^{2}}$ hybridized.
From the above diagram it is evident that option D is the correct answer.
Note: Knowing the valance electrons of a particular element may help us to figure out their structure. Always whenever, a double bond is formed. Here one of the bonds will be $\sigma $ bond and the other will be $\pi $ bond. Now, the $\pi $ bond can be p\[\pi \]-p\[\pi \]bond, d$\pi $- p$\pi $bond or d\[\pi \]-d\[\pi \]bond.
Complete answer:
Sulphur has six electrons in its outermost shell that is its covalency is six. Covalency of an atom is the number bonds an atom can make. In solid state the $S{{O}_{3}}$molecule exists in a cluster that is the number of$S{{O}_{3}}$ molecules bonded together to form a solid structure. In the gas phase the $S{{O}_{3}}$ molecule exists as a single molecule which is also said to exist in the free State.
$\sigma $- bond is formed by overlapping of atomic orbitals or hybrid orbitals in their axis.
For example: overlap of s-s, s-p,$s{{p}^{2}}$-$s{{p}^{2}}$ overlap along their axis.
\[\pi \]- bond is formed by the side to side overlap of molecular orbital or atomic orbital along a plane perpendicular to the plane. If the donating orbital is p and the orbital to which the electron pair is donated is p then it is known as P\[\pi \]-p\[\pi \].
Similarly, if the donating orbital is d and the orbital to which the electron pair is donated is p then it is known as d$\pi $- p$\pi $
Firstly, let's draw the Lewis structure of$S{{O}_{3}}$:

There are three delocalised \[\pi \] bonds, out of which one of them is P\[\pi \]-p\[\pi \]and other two are d$\pi $- p$\pi $. The sulphur is $d\pi -p\pi $hybridized.
The electronic configuration of sulphur is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{4}}$.
Sulphur in ground state:

In excited state:

Oxygen has 6 electrons. So, the valence electrons of oxygen make bond with unpaired electrons.

The 6 valence electrons in oxygen are of 2p orbital.

The red coloured electrons in the above mentioned diagram are the electrons of oxygen. The $s{{p}^{2}}$-$s{{p}^{2}}$ overlap forms $\sigma $ bond as sulphur in $S{{O}_{3}}$ is $s{{p}^{2}}$ hybridized.
From the above diagram it is evident that option D is the correct answer.
Note: Knowing the valance electrons of a particular element may help us to figure out their structure. Always whenever, a double bond is formed. Here one of the bonds will be $\sigma $ bond and the other will be $\pi $ bond. Now, the $\pi $ bond can be p\[\pi \]-p\[\pi \]bond, d$\pi $- p$\pi $bond or d\[\pi \]-d\[\pi \]bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




