
The structural formula of iso-butene is:
A. $\text{C}{{\text{H}}_{3}}\text{C}{{\text{H}}_{2}}\text{CH = C}{{\text{H}}_{2}}$
B. $\text{C}{{\text{H}}_{3}}\text{CH = CH - C}{{\text{H}}_{3}}$
C. $\text{C}{{\text{H}}_{3}}\text{CH(C}{{\text{H}}_{3}}\text{) - C}{{\text{H}}_{3}}$
D. $\text{C}{{\text{H}}_{2}}\text{ = C(C}{{\text{H}}_{3}})\text{ - C}{{\text{H}}_{3}}$
Answer
582.9k+ views
Hint: For this problem, we have to study about 'iso' structure and how much branching takes place in it. After which we will be able to choose the structural formula of iso-butene in which the one double bond is also present at the first carbon.
Complete step by step answer:
- In the given question, we have to choose the correct structural formula of iso-butene among the given options.
- The word 'iso' is used as a prefix in which the molecule has all the carbons in the straight-chain except one carbon.
- In iso, one carbon is present on the 2 - carbon atom forming a branched structure.
- Now, as we know that the prefix 'but' is used when there are four carbon atoms present in the chain and 'ene' is used for the presence of double bond.
- So, the structural formula of the iso-butene will be:
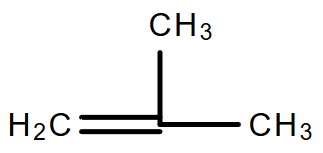
- So, as we can see that in the first option the chain is straight with no branching so it will be the incorrect answer.
- Whereas in the second option also the chain is straight without branching so iso cannot be used for the structure and this option is also incorrect.
- But in the third option branching is present which is correct but due to the absence of the double bond, it will also be the incorrect answer.
So, the correct answer is “Option D”.
Note: Other than iso, the prefix neo is also used for the more branched structure. In neo, two carbon atoms attach on the second carbon. Whereas just like ene is used for the double bond, the prefix 'ane' is used for a single bond.
Complete step by step answer:
- In the given question, we have to choose the correct structural formula of iso-butene among the given options.
- The word 'iso' is used as a prefix in which the molecule has all the carbons in the straight-chain except one carbon.
- In iso, one carbon is present on the 2 - carbon atom forming a branched structure.
- Now, as we know that the prefix 'but' is used when there are four carbon atoms present in the chain and 'ene' is used for the presence of double bond.
- So, the structural formula of the iso-butene will be:

- So, as we can see that in the first option the chain is straight with no branching so it will be the incorrect answer.
- Whereas in the second option also the chain is straight without branching so iso cannot be used for the structure and this option is also incorrect.
- But in the third option branching is present which is correct but due to the absence of the double bond, it will also be the incorrect answer.
So, the correct answer is “Option D”.
Note: Other than iso, the prefix neo is also used for the more branched structure. In neo, two carbon atoms attach on the second carbon. Whereas just like ene is used for the double bond, the prefix 'ane' is used for a single bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




