
The reagent(s) used in the preparation of aspirin from salicylic acid is:
(a) ${ SOCl }_{ 2 }$, pyridine
(b) ${ (CH }_{ 3 }CO{ ) }_{ 2 }O$, ${ H }^{ + }$
(C) ${ CH }_{ 3 }{ CO }_{ 2 }H$, HCl
(d) ${ CH }_{ 3 }Cl$, $Al{ Cl }_{ 3 }$
Answer
593.1k+ views
Hint: Aspirin is 2-Acetoxybenzoic acid which is prepared from Salicylic acid (2-Hydroxybenzoic acid) as the starting material. Aspirin is a non-narcotic analgesic which is used as antipyretic and as a pain reliever.
Complete step by step answer:
Aspirin is also called 2-acetoxy benzoic acid. It acts as an analgesic. An analgesic is a drug that reduces or relieves pain. They are classified as non-narcotic or narcotic pain relievers. Aspirin is non-narcotic i.e. non-opioid and is used to reduce pain and inflammation. It is also antipyretic.
We can easily prepare aspirin by acetylating salicylic acid using acetic anhydride in the presence of concentrated $ { H }_{ 2 }{ SO }_{ 4 }$.
For synthesising aspirin, we first need to prepare salicylic acid. Salicylic acid is ortho-hydroxybenzoic acid. It can be synthesised using the Kolbe-Schmitt process. The first step involves the reaction of phenol and sodium hydroxide in order to produce sodium phenoxide. This phenoxide is then reacted with carbon dioxide to form sodium salicylate which on acidification gives salicylic acid. The reactions are given below:
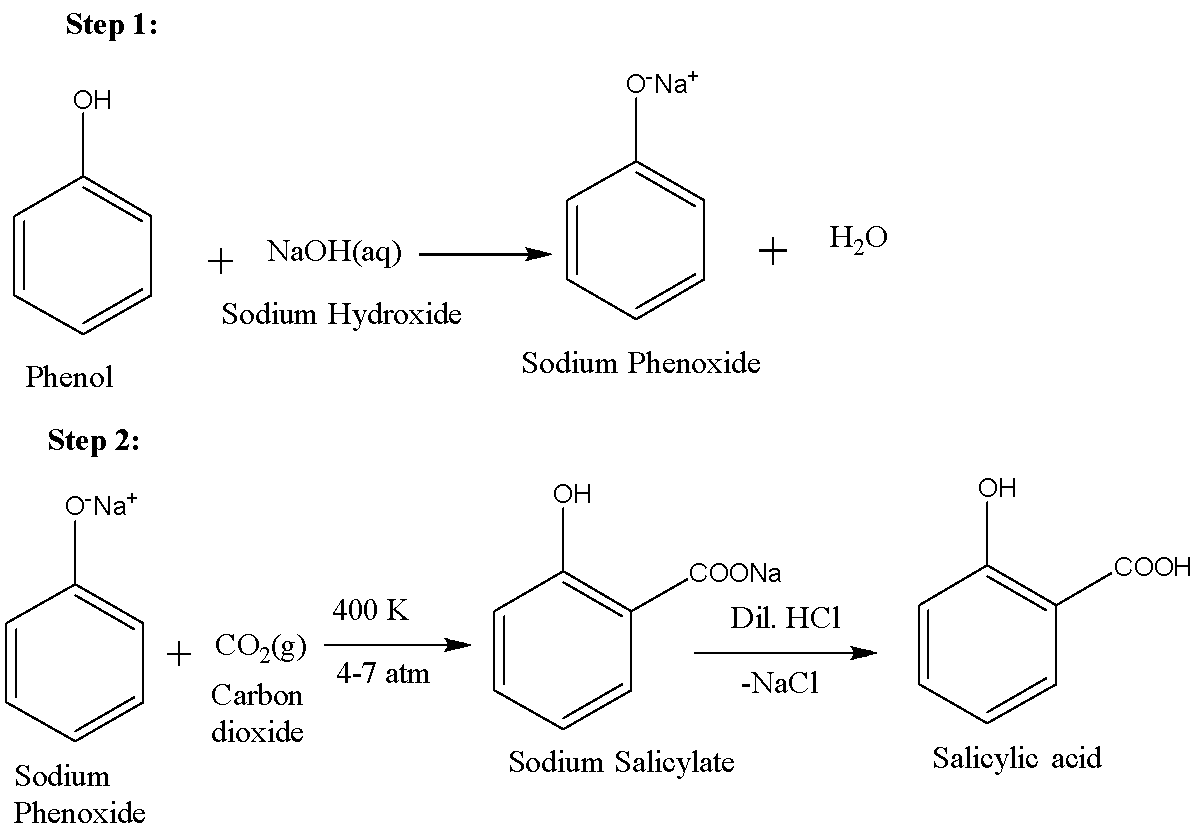
Now using this Salicylic acid, we will prepare aspirin by acetylation. The reactions are given below:
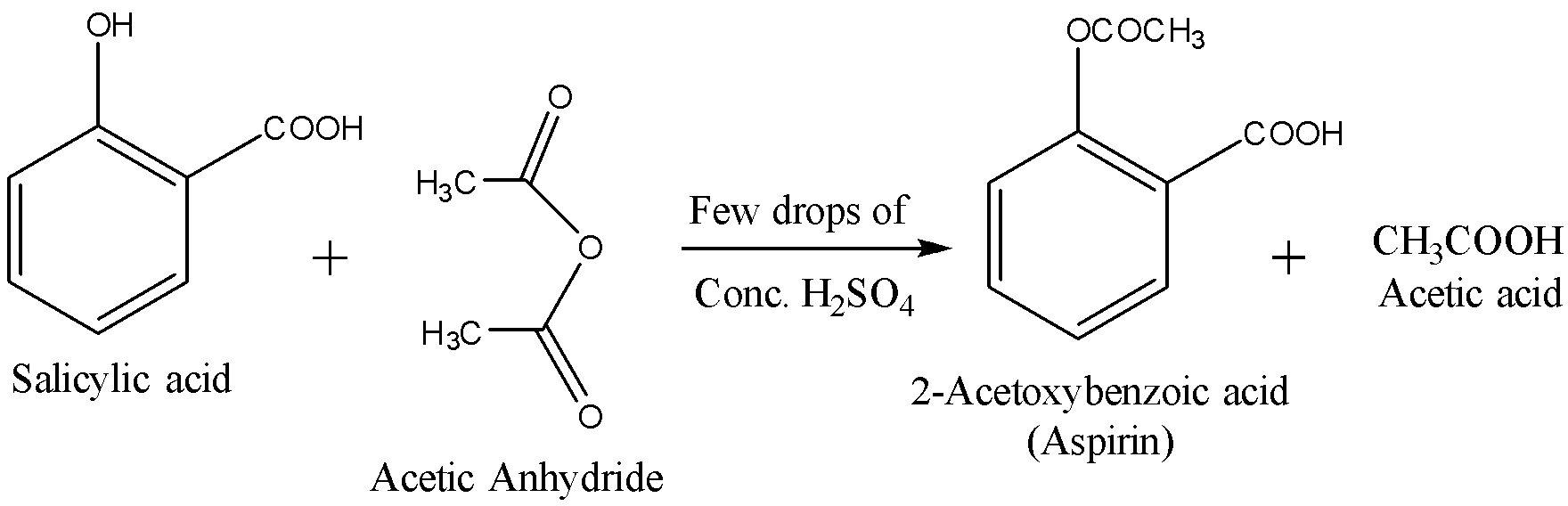
So, the correct answer is “Option (B) ${ (CH }_{ 3 }CO{ ) }_{ 2 }O$, ${ H }^{ + }$”.
Note: All the above reactions are taking place in an aqueous medium. The end product i.e. Aspirin is also crystallised from the aqueous medium. The Kolbe-Schmitt process is a very old process, though still in use, many new efficient methods are used for the manufacture of Aspirin now.
Complete step by step answer:
Aspirin is also called 2-acetoxy benzoic acid. It acts as an analgesic. An analgesic is a drug that reduces or relieves pain. They are classified as non-narcotic or narcotic pain relievers. Aspirin is non-narcotic i.e. non-opioid and is used to reduce pain and inflammation. It is also antipyretic.
We can easily prepare aspirin by acetylating salicylic acid using acetic anhydride in the presence of concentrated $ { H }_{ 2 }{ SO }_{ 4 }$.
For synthesising aspirin, we first need to prepare salicylic acid. Salicylic acid is ortho-hydroxybenzoic acid. It can be synthesised using the Kolbe-Schmitt process. The first step involves the reaction of phenol and sodium hydroxide in order to produce sodium phenoxide. This phenoxide is then reacted with carbon dioxide to form sodium salicylate which on acidification gives salicylic acid. The reactions are given below:

Now using this Salicylic acid, we will prepare aspirin by acetylation. The reactions are given below:

So, the correct answer is “Option (B) ${ (CH }_{ 3 }CO{ ) }_{ 2 }O$, ${ H }^{ + }$”.
Note: All the above reactions are taking place in an aqueous medium. The end product i.e. Aspirin is also crystallised from the aqueous medium. The Kolbe-Schmitt process is a very old process, though still in use, many new efficient methods are used for the manufacture of Aspirin now.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




