
The reaction of PhOH with aldehyde RCHO in acidic medium gives:
Answer
519k+ views
Hint: We know that phenol is an aromatic organic compound with the molecular formula. In biochemistry, an aldehyde is a major functional group with the general formula RCHO. This formula consists of a carbonyl functional group, which uses the carbon's two remaining bonds to bind to hydrogen, and an R functional group, which may be second hydrogen, an alkyl, or an aryl group.
Complete answer:
We know that, PhOH is ortho directing and para directing. So, in acidic medium, the aldehyde RCHO is bound to ortho and para positions. Because of steric hindrance, the ortho product is less durable, and the para product is the most common.
This reaction can be represented as:
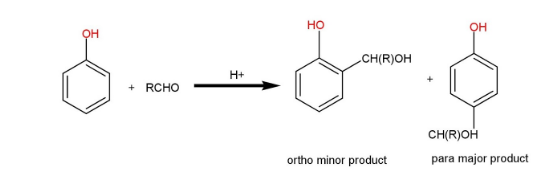
We can define this reaction as, under neutral conditions alcohols are relatively weaker nucleophiles and bind steadily to the group. However, in an acidic medium, the velocity increases as the group protonates, raising the positive charge on the carbonyl C atom and making it more reactive. Since \[R{O^ - }\] is in equilibrium with OH in simple medium and reacts with the group faster than ROH. Furthermore, \[R{O^ - }\] is a strong nucleophile.
Hence, we can conclude that, para product is the most common in this reaction.
Note:
We should remember that the electron density in ortho is lower than in para. As a result, ortho is a minor commodity and para is a main product. Also, the para compound is usually the main product when an ortho-para directing substituent is present on the benzene ring for an electrophilic aromatic substitution reaction.
Complete answer:
We know that, PhOH is ortho directing and para directing. So, in acidic medium, the aldehyde RCHO is bound to ortho and para positions. Because of steric hindrance, the ortho product is less durable, and the para product is the most common.
This reaction can be represented as:

We can define this reaction as, under neutral conditions alcohols are relatively weaker nucleophiles and bind steadily to the group. However, in an acidic medium, the velocity increases as the group protonates, raising the positive charge on the carbonyl C atom and making it more reactive. Since \[R{O^ - }\] is in equilibrium with OH in simple medium and reacts with the group faster than ROH. Furthermore, \[R{O^ - }\] is a strong nucleophile.
Hence, we can conclude that, para product is the most common in this reaction.
Note:
We should remember that the electron density in ortho is lower than in para. As a result, ortho is a minor commodity and para is a main product. Also, the para compound is usually the main product when an ortho-para directing substituent is present on the benzene ring for an electrophilic aromatic substitution reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




